Zinc »
PDB 8i3n-8ihq »
8ihq »
Zinc in PDB 8ihq: Cryo-Em Structure of Ochratoxin A-Detoxifying Amidohydrolase ADH3
Zinc Binding Sites:
Pages:
>>> Page 1 <<< Page 2, Binding sites: 11 - 16;Binding sites:
The binding sites of Zinc atom in the Cryo-Em Structure of Ochratoxin A-Detoxifying Amidohydrolase ADH3 (pdb code 8ihq). This binding sites where shown within 5.0 Angstroms radius around Zinc atom.In total 16 binding sites of Zinc where determined in the Cryo-Em Structure of Ochratoxin A-Detoxifying Amidohydrolase ADH3, PDB code: 8ihq:
Jump to Zinc binding site number: 1; 2; 3; 4; 5; 6; 7; 8; 9; 10;
Zinc binding site 1 out of 16 in 8ihq
Go back to
Zinc binding site 1 out
of 16 in the Cryo-Em Structure of Ochratoxin A-Detoxifying Amidohydrolase ADH3
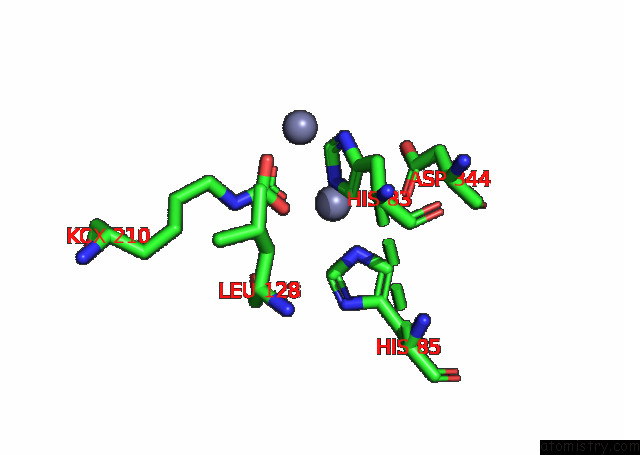
Mono view
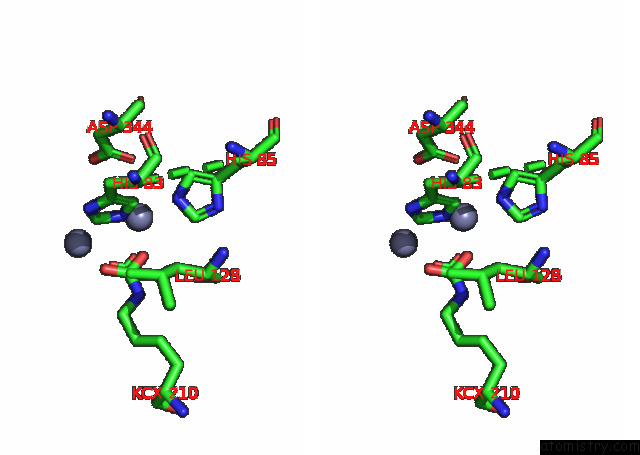
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Cryo-Em Structure of Ochratoxin A-Detoxifying Amidohydrolase ADH3 within 5.0Å range:
|
Zinc binding site 2 out of 16 in 8ihq
Go back to
Zinc binding site 2 out
of 16 in the Cryo-Em Structure of Ochratoxin A-Detoxifying Amidohydrolase ADH3
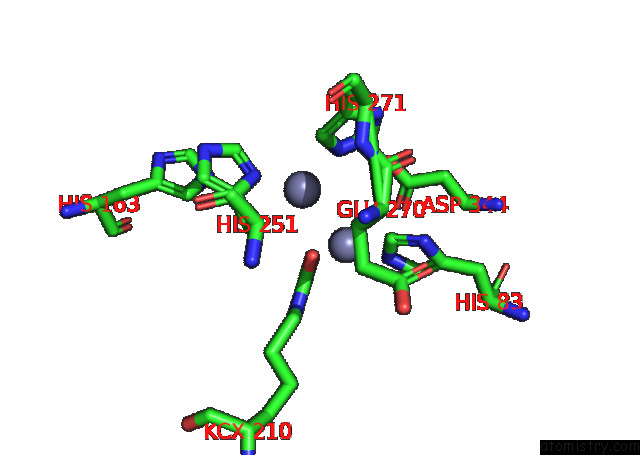
Mono view
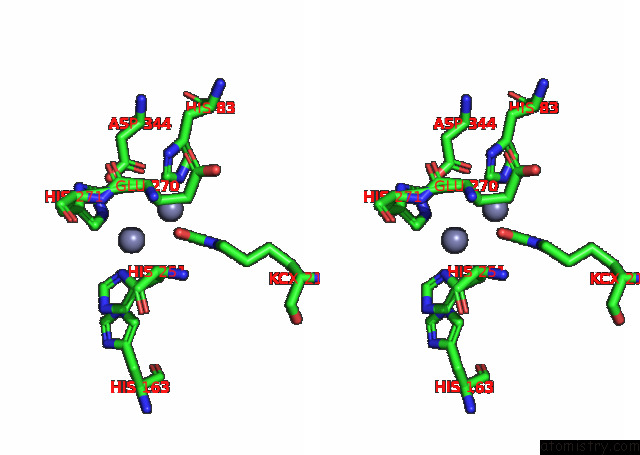
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of Cryo-Em Structure of Ochratoxin A-Detoxifying Amidohydrolase ADH3 within 5.0Å range:
|
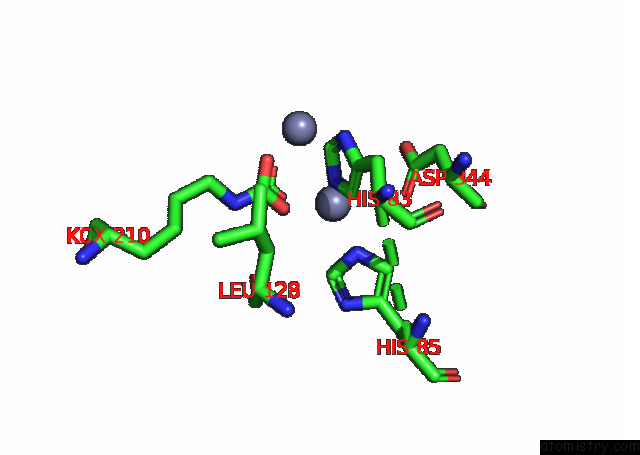
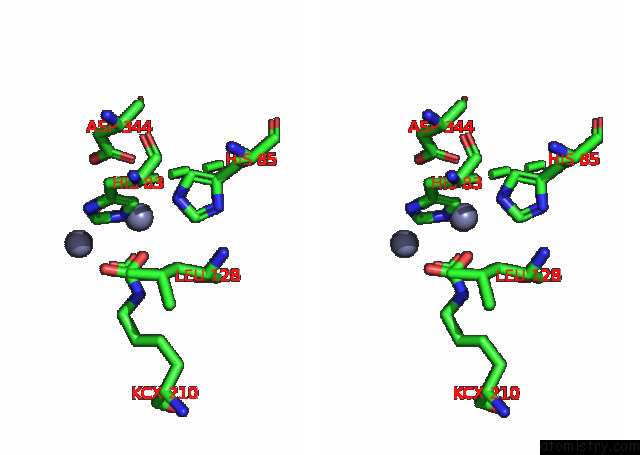
Zinc binding site 3 out of 16 in 8ihq
Go back to
Zinc binding site 3 out
of 16 in the Cryo-Em Structure of Ochratoxin A-Detoxifying Amidohydrolase ADH3
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 3 of Cryo-Em Structure of Ochratoxin A-Detoxifying Amidohydrolase ADH3 within 5.0Å range:
|
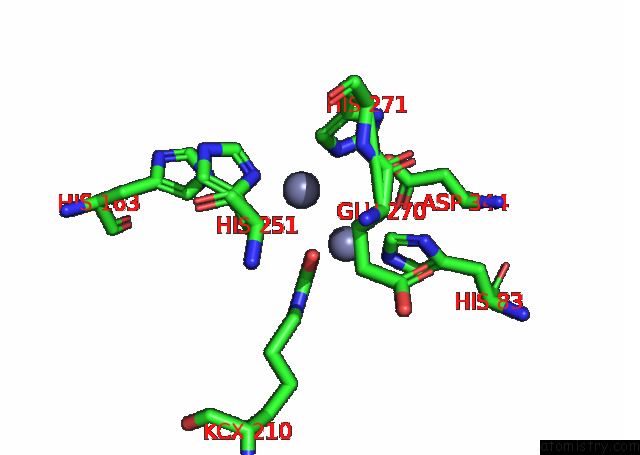
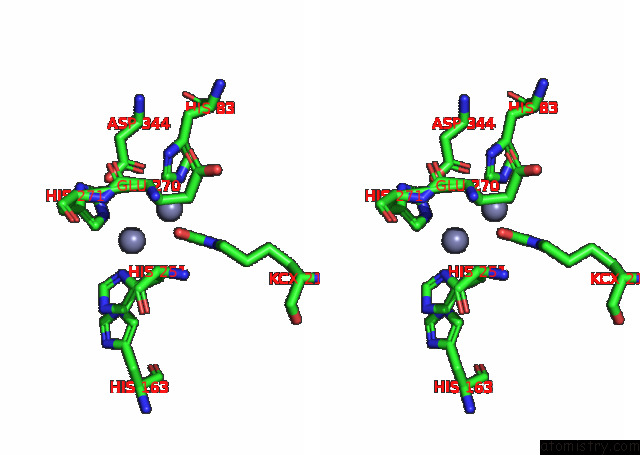
Zinc binding site 4 out of 16 in 8ihq
Go back to
Zinc binding site 4 out
of 16 in the Cryo-Em Structure of Ochratoxin A-Detoxifying Amidohydrolase ADH3
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 4 of Cryo-Em Structure of Ochratoxin A-Detoxifying Amidohydrolase ADH3 within 5.0Å range:
|
Zinc binding site 5 out of 16 in 8ihq
Go back to
Zinc binding site 5 out
of 16 in the Cryo-Em Structure of Ochratoxin A-Detoxifying Amidohydrolase ADH3
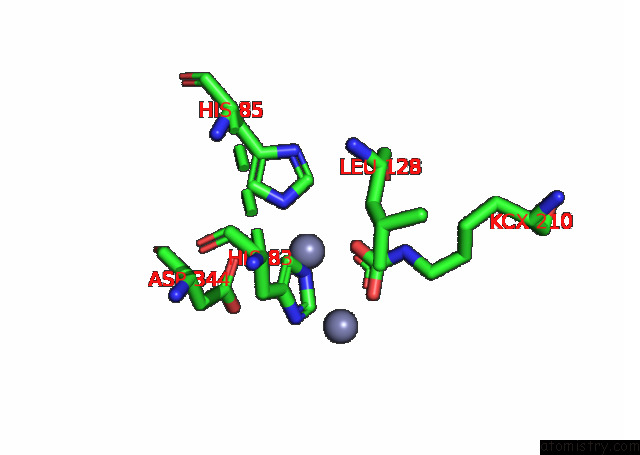
Mono view
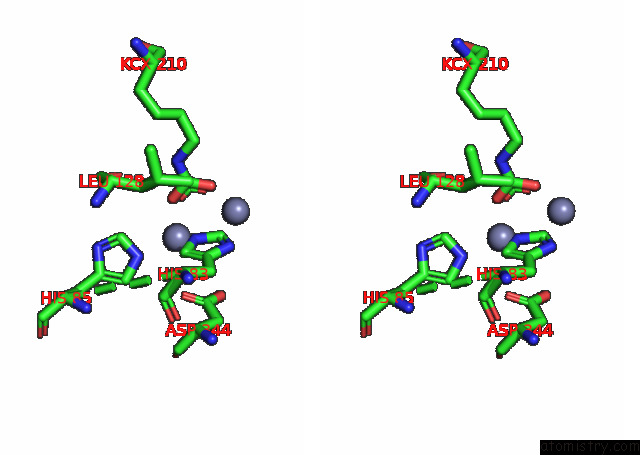
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 5 of Cryo-Em Structure of Ochratoxin A-Detoxifying Amidohydrolase ADH3 within 5.0Å range:
|
Zinc binding site 6 out of 16 in 8ihq
Go back to
Zinc binding site 6 out
of 16 in the Cryo-Em Structure of Ochratoxin A-Detoxifying Amidohydrolase ADH3
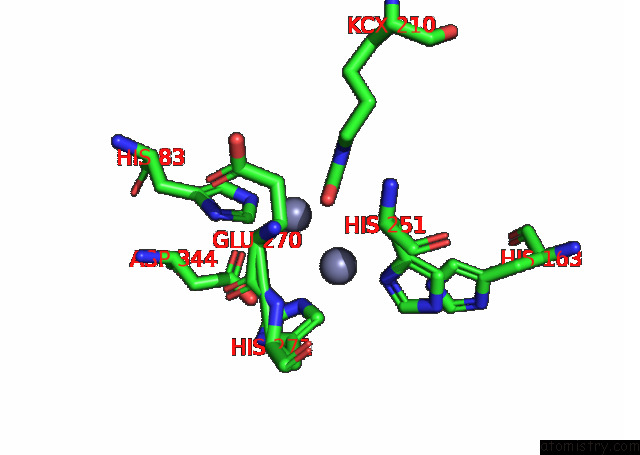
Mono view
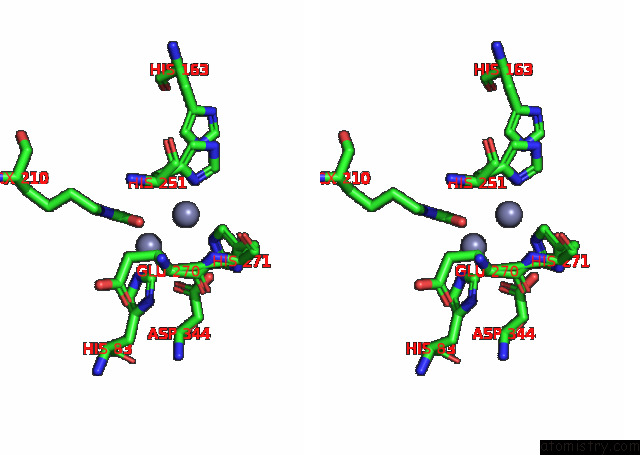
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 6 of Cryo-Em Structure of Ochratoxin A-Detoxifying Amidohydrolase ADH3 within 5.0Å range:
|
Zinc binding site 7 out of 16 in 8ihq
Go back to
Zinc binding site 7 out
of 16 in the Cryo-Em Structure of Ochratoxin A-Detoxifying Amidohydrolase ADH3
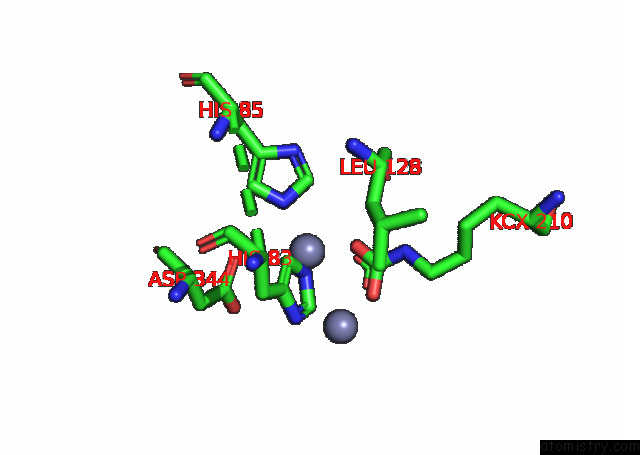
Mono view
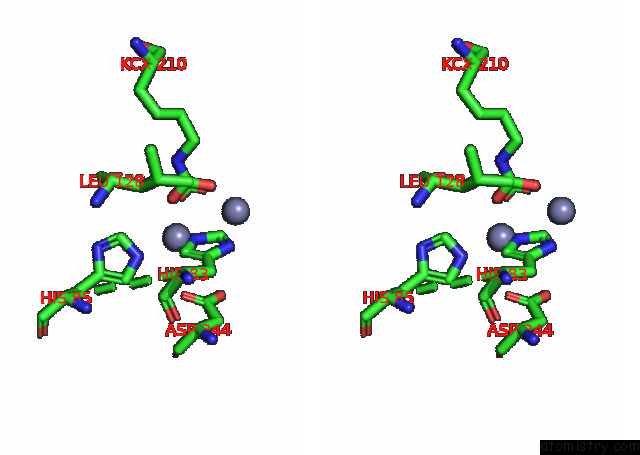
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 7 of Cryo-Em Structure of Ochratoxin A-Detoxifying Amidohydrolase ADH3 within 5.0Å range:
|
Zinc binding site 8 out of 16 in 8ihq
Go back to
Zinc binding site 8 out
of 16 in the Cryo-Em Structure of Ochratoxin A-Detoxifying Amidohydrolase ADH3
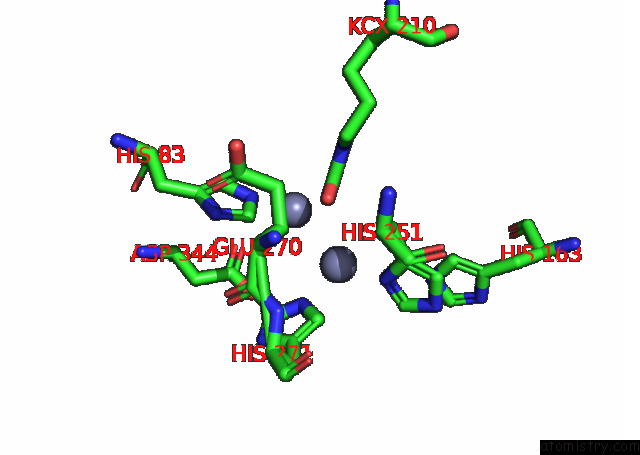
Mono view
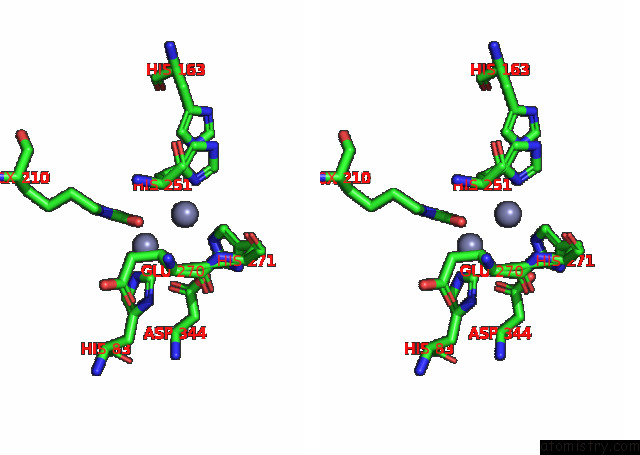
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 8 of Cryo-Em Structure of Ochratoxin A-Detoxifying Amidohydrolase ADH3 within 5.0Å range:
|
Zinc binding site 9 out of 16 in 8ihq
Go back to
Zinc binding site 9 out
of 16 in the Cryo-Em Structure of Ochratoxin A-Detoxifying Amidohydrolase ADH3
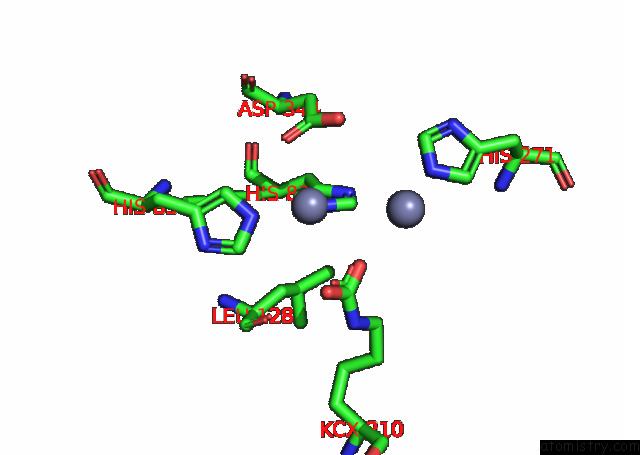
Mono view
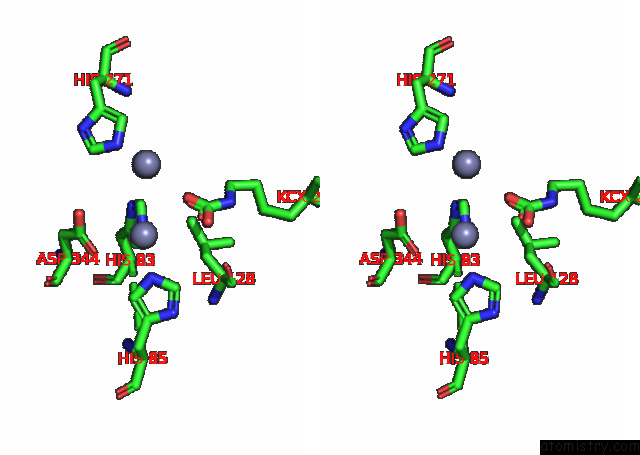
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 9 of Cryo-Em Structure of Ochratoxin A-Detoxifying Amidohydrolase ADH3 within 5.0Å range:
|
Zinc binding site 10 out of 16 in 8ihq
Go back to
Zinc binding site 10 out
of 16 in the Cryo-Em Structure of Ochratoxin A-Detoxifying Amidohydrolase ADH3
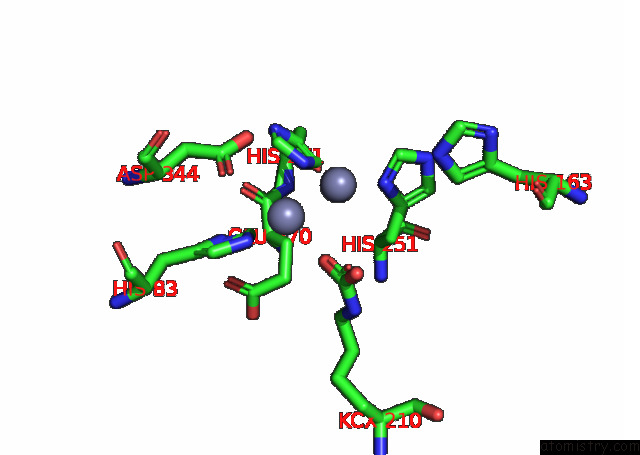
Mono view
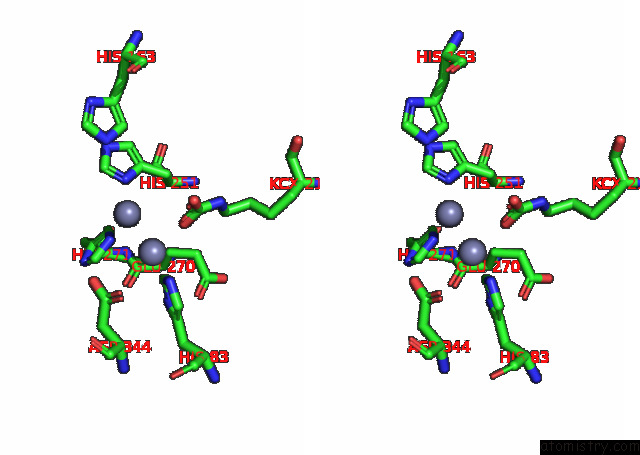
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 10 of Cryo-Em Structure of Ochratoxin A-Detoxifying Amidohydrolase ADH3 within 5.0Å range:
|
Reference:
L.Dai,
D.Niu,
J.W.Huang,
X.Li,
P.Shen,
H.Li,
Z.Xie,
J.Min,
Y.Hu,
Y.Yang,
R.T.Guo,
C.C.Chen.
Cryo-Em Structure and Rational Engineering of A Superefficient Ochratoxin A-Detoxifying Amidohydrolase. J Hazard Mater V. 458 31836 2023.
ISSN: ESSN 1873-3336
PubMed: 37331057
DOI: 10.1016/J.JHAZMAT.2023.131836
Page generated: Thu Oct 31 07:46:40 2024
ISSN: ESSN 1873-3336
PubMed: 37331057
DOI: 10.1016/J.JHAZMAT.2023.131836
Last articles
Zn in 9JYWZn in 9IR4
Zn in 9IR3
Zn in 9GMX
Zn in 9GMW
Zn in 9JEJ
Zn in 9ERF
Zn in 9ERE
Zn in 9EGV
Zn in 9EGW