Zinc »
PDB 5zn8-6a12 »
5zx3 »
Zinc in PDB 5zx3: Mycobacterium Tuberculosis Rna Polymerase Holoenzyme with Ecf Sigma Factor Sigma H
Enzymatic activity of Mycobacterium Tuberculosis Rna Polymerase Holoenzyme with Ecf Sigma Factor Sigma H
All present enzymatic activity of Mycobacterium Tuberculosis Rna Polymerase Holoenzyme with Ecf Sigma Factor Sigma H:
2.7.7.6;
2.7.7.6;
Protein crystallography data
The structure of Mycobacterium Tuberculosis Rna Polymerase Holoenzyme with Ecf Sigma Factor Sigma H, PDB code: 5zx3
was solved by
L.Li,
Y.Zhang,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 41.64 / 2.75 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 130.572, 159.790, 131.437, 90.00, 118.72, 90.00 |
| R / Rfree (%) | 21.8 / 25.8 |
Zinc Binding Sites:
The binding sites of Zinc atom in the Mycobacterium Tuberculosis Rna Polymerase Holoenzyme with Ecf Sigma Factor Sigma H
(pdb code 5zx3). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 2 binding sites of Zinc where determined in the Mycobacterium Tuberculosis Rna Polymerase Holoenzyme with Ecf Sigma Factor Sigma H, PDB code: 5zx3:
Jump to Zinc binding site number: 1; 2;
In total 2 binding sites of Zinc where determined in the Mycobacterium Tuberculosis Rna Polymerase Holoenzyme with Ecf Sigma Factor Sigma H, PDB code: 5zx3:
Jump to Zinc binding site number: 1; 2;
Zinc binding site 1 out of 2 in 5zx3
Go back to
Zinc binding site 1 out
of 2 in the Mycobacterium Tuberculosis Rna Polymerase Holoenzyme with Ecf Sigma Factor Sigma H
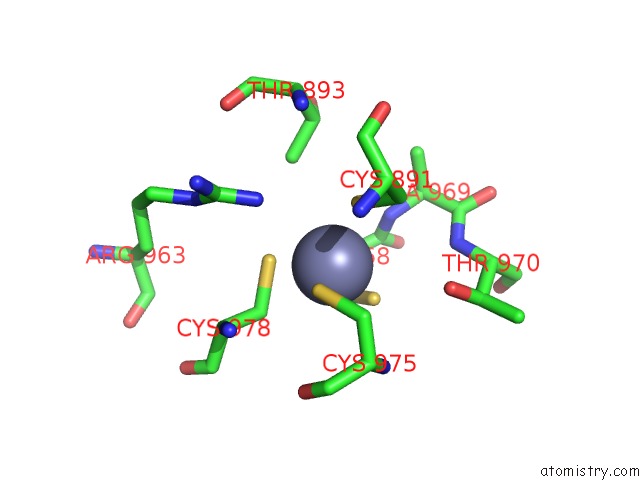
Mono view
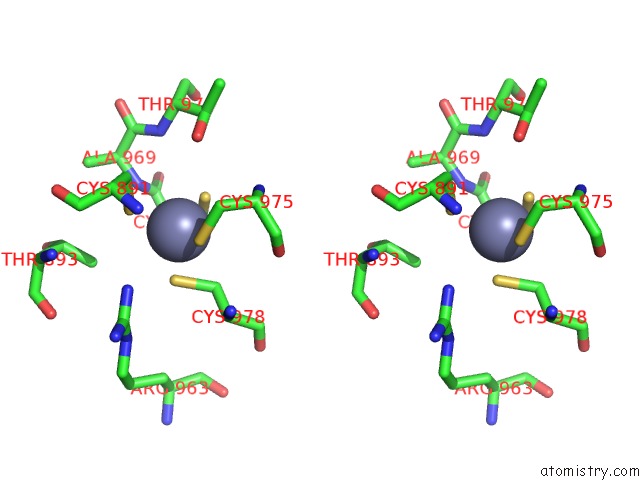
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Mycobacterium Tuberculosis Rna Polymerase Holoenzyme with Ecf Sigma Factor Sigma H within 5.0Å range:
|
Zinc binding site 2 out of 2 in 5zx3
Go back to
Zinc binding site 2 out
of 2 in the Mycobacterium Tuberculosis Rna Polymerase Holoenzyme with Ecf Sigma Factor Sigma H
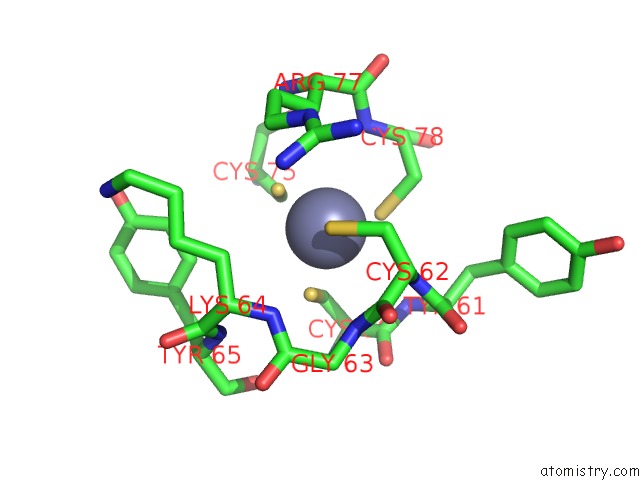
Mono view
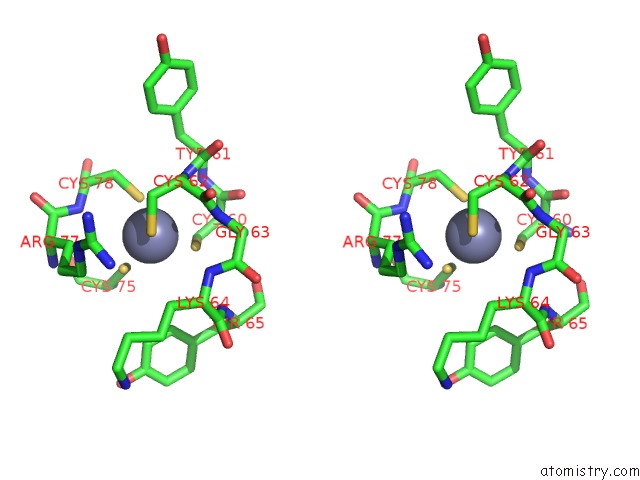
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of Mycobacterium Tuberculosis Rna Polymerase Holoenzyme with Ecf Sigma Factor Sigma H within 5.0Å range:
|
Reference:
L.Li,
C.Fang,
N.Zhuang,
T.Wang,
Y.Zhang.
Structural Basis For Transcription Initiation By Bacterial Ecf Sigma Factors. Nat Commun V. 10 1153 2019.
ISSN: ESSN 2041-1723
PubMed: 30858373
DOI: 10.1038/S41467-019-09096-Y
Page generated: Mon Oct 28 17:11:24 2024
ISSN: ESSN 2041-1723
PubMed: 30858373
DOI: 10.1038/S41467-019-09096-Y
Last articles
Zn in 9JYWZn in 9IR4
Zn in 9IR3
Zn in 9GMX
Zn in 9GMW
Zn in 9JEJ
Zn in 9ERF
Zn in 9ERE
Zn in 9EGV
Zn in 9EGW