Zinc »
PDB 5mcu-5msa »
5mnr »
Zinc in PDB 5mnr: Thermolysin in Complex with Inhibitor JC256
Enzymatic activity of Thermolysin in Complex with Inhibitor JC256
All present enzymatic activity of Thermolysin in Complex with Inhibitor JC256:
3.4.24.27;
3.4.24.27;
Protein crystallography data
The structure of Thermolysin in Complex with Inhibitor JC256, PDB code: 5mnr
was solved by
J.Cramer,
S.G.Krimmer,
A.Heine,
G.Klebe,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 40.16 / 1.25 |
| Space group | P 61 2 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 92.735, 92.735, 130.538, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 10.3 / 12.7 |
Other elements in 5mnr:
The structure of Thermolysin in Complex with Inhibitor JC256 also contains other interesting chemical elements:
| Calcium | (Ca) | 4 atoms |
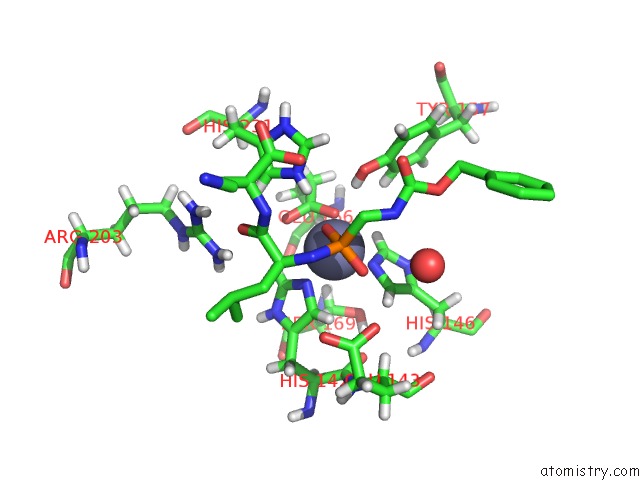
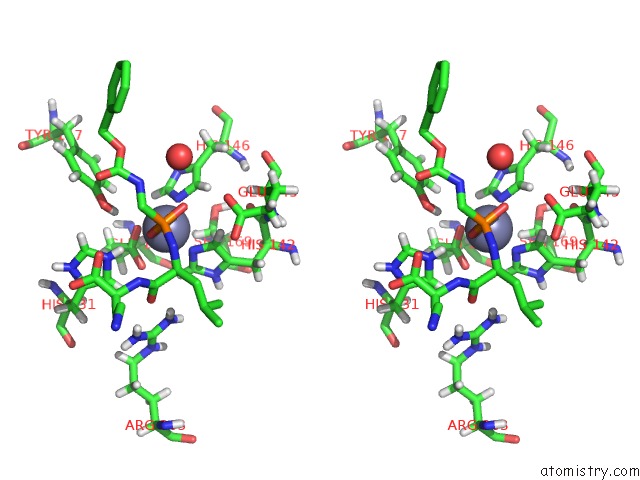
Zinc Binding Sites:
The binding sites of Zinc atom in the Thermolysin in Complex with Inhibitor JC256
(pdb code 5mnr). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total only one binding site of Zinc was determined in the Thermolysin in Complex with Inhibitor JC256, PDB code: 5mnr:
In total only one binding site of Zinc was determined in the Thermolysin in Complex with Inhibitor JC256, PDB code: 5mnr:
Zinc binding site 1 out of 1 in 5mnr
Go back to
Zinc binding site 1 out
of 1 in the Thermolysin in Complex with Inhibitor JC256
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Thermolysin in Complex with Inhibitor JC256 within 5.0Å range:
|
Reference:
J.Cramer,
S.G.Krimmer,
A.Heine,
G.Klebe.
Paying the Price of Desolvation in Solvent-Exposed Protein Pockets: Impact of Distal Solubilizing Groups on Affinity and Binding Thermodynamics in A Series of Thermolysin Inhibitors. J. Med. Chem. V. 60 5791 2017.
ISSN: ISSN 1520-4804
PubMed: 28590130
DOI: 10.1021/ACS.JMEDCHEM.7B00490
Page generated: Sun Oct 27 22:13:19 2024
ISSN: ISSN 1520-4804
PubMed: 28590130
DOI: 10.1021/ACS.JMEDCHEM.7B00490
Last articles
Zn in 9JYWZn in 9IR4
Zn in 9IR3
Zn in 9GMX
Zn in 9GMW
Zn in 9JEJ
Zn in 9ERF
Zn in 9ERE
Zn in 9EGV
Zn in 9EGW