Zinc »
PDB 2m0f-2mlr »
2m5c »
Zinc in PDB 2m5c: Solution Structure of the Bacillus Cereus Metallo-Beta-Lactamase Bcii
Enzymatic activity of Solution Structure of the Bacillus Cereus Metallo-Beta-Lactamase Bcii
All present enzymatic activity of Solution Structure of the Bacillus Cereus Metallo-Beta-Lactamase Bcii:
3.5.2.6;
3.5.2.6;
Zinc Binding Sites:
The binding sites of Zinc atom in the Solution Structure of the Bacillus Cereus Metallo-Beta-Lactamase Bcii
(pdb code 2m5c). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 2 binding sites of Zinc where determined in the Solution Structure of the Bacillus Cereus Metallo-Beta-Lactamase Bcii, PDB code: 2m5c:
Jump to Zinc binding site number: 1; 2;
In total 2 binding sites of Zinc where determined in the Solution Structure of the Bacillus Cereus Metallo-Beta-Lactamase Bcii, PDB code: 2m5c:
Jump to Zinc binding site number: 1; 2;
Zinc binding site 1 out of 2 in 2m5c
Go back to
Zinc binding site 1 out
of 2 in the Solution Structure of the Bacillus Cereus Metallo-Beta-Lactamase Bcii
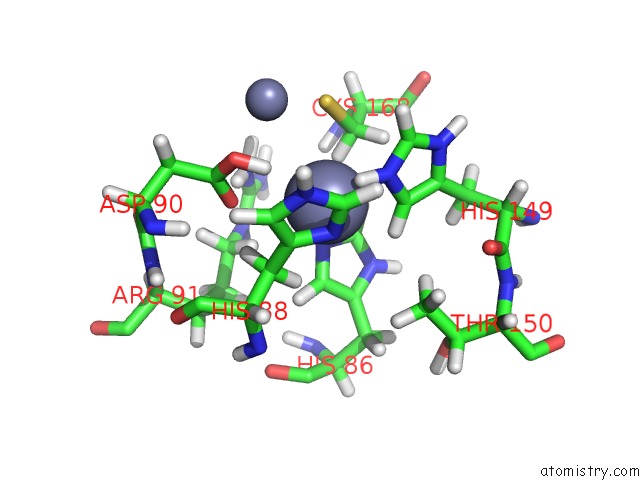
Mono view
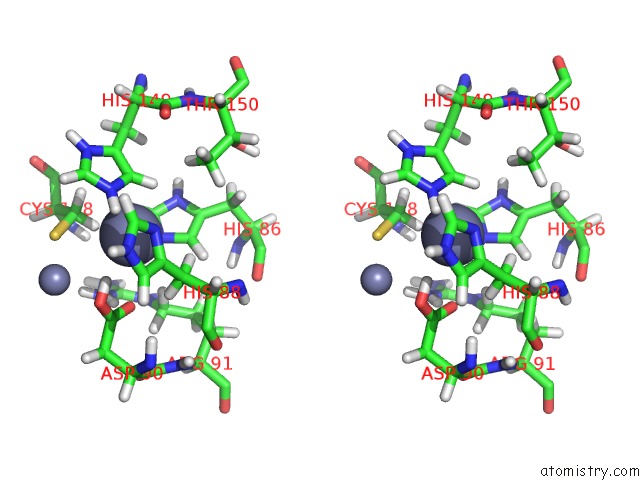
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Solution Structure of the Bacillus Cereus Metallo-Beta-Lactamase Bcii within 5.0Å range:
|
Zinc binding site 2 out of 2 in 2m5c
Go back to
Zinc binding site 2 out
of 2 in the Solution Structure of the Bacillus Cereus Metallo-Beta-Lactamase Bcii
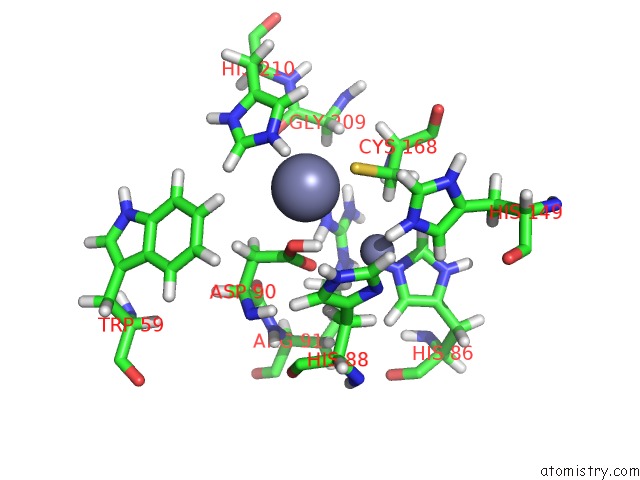
Mono view
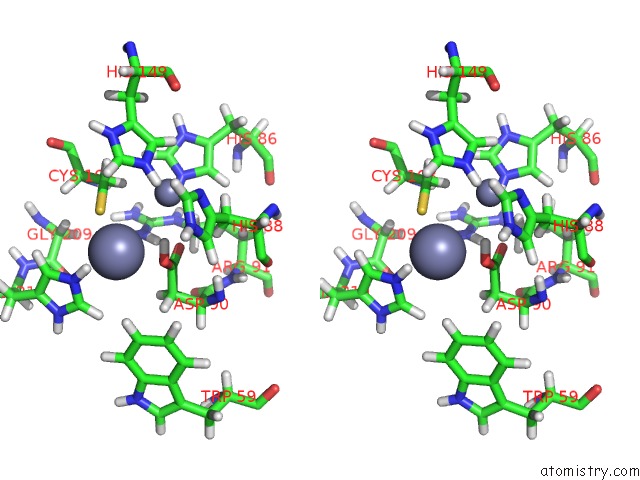
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of Solution Structure of the Bacillus Cereus Metallo-Beta-Lactamase Bcii within 5.0Å range:
|
Reference:
A.I.Karsisiotis,
C.F.Damblon,
G.C.K.Roberts.
Solution Structures of the Bacillus Cereus Metallo-Beta-Lactamase Bcii and Its Complex with the Broad Spectrum Inhibitor R-Thiomandelic Acid Biochem.J. 2013.
ISSN: ESSN 1470-8728
PubMed: 24059435
DOI: 10.1042/BJ20131003
Page generated: Thu Oct 17 02:00:05 2024
ISSN: ESSN 1470-8728
PubMed: 24059435
DOI: 10.1042/BJ20131003
Last articles
Zn in 9JYWZn in 9IR4
Zn in 9IR3
Zn in 9GMX
Zn in 9GMW
Zn in 9JEJ
Zn in 9ERF
Zn in 9ERE
Zn in 9EGV
Zn in 9EGW