Zinc »
PDB 2lgk-2m0e »
2lgk »
Zinc in PDB 2lgk: uc(Nmr) Structure of UHRF1 Phd Domains in A Complex with Histone H3 Peptide
Zinc Binding Sites:
The binding sites of Zinc atom in the uc(Nmr) Structure of UHRF1 Phd Domains in A Complex with Histone H3 Peptide
(pdb code 2lgk). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 3 binding sites of Zinc where determined in the uc(Nmr) Structure of UHRF1 Phd Domains in A Complex with Histone H3 Peptide, PDB code: 2lgk:
Jump to Zinc binding site number: 1; 2; 3;
In total 3 binding sites of Zinc where determined in the uc(Nmr) Structure of UHRF1 Phd Domains in A Complex with Histone H3 Peptide, PDB code: 2lgk:
Jump to Zinc binding site number: 1; 2; 3;
Zinc binding site 1 out of 3 in 2lgk
Go back to
Zinc binding site 1 out
of 3 in the uc(Nmr) Structure of UHRF1 Phd Domains in A Complex with Histone H3 Peptide
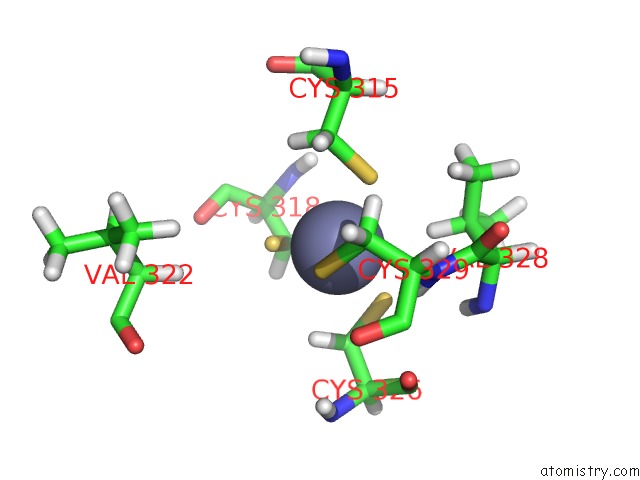
Mono view
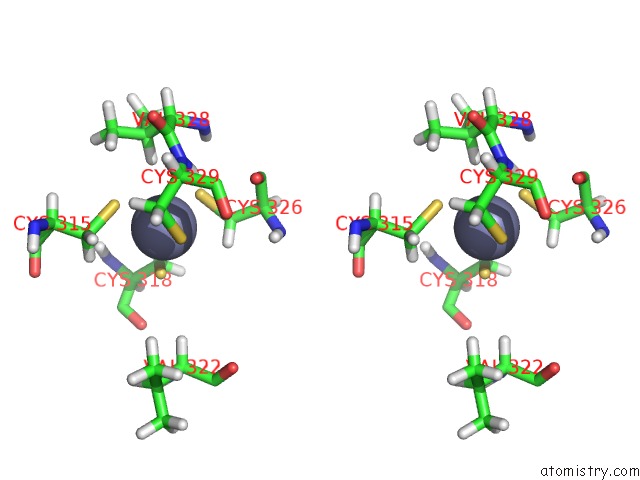
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of uc(Nmr) Structure of UHRF1 Phd Domains in A Complex with Histone H3 Peptide within 5.0Å range:
|
Zinc binding site 2 out of 3 in 2lgk
Go back to
Zinc binding site 2 out
of 3 in the uc(Nmr) Structure of UHRF1 Phd Domains in A Complex with Histone H3 Peptide
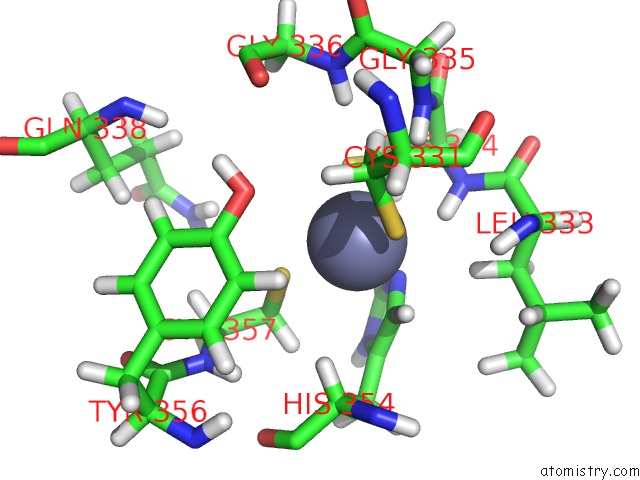
Mono view
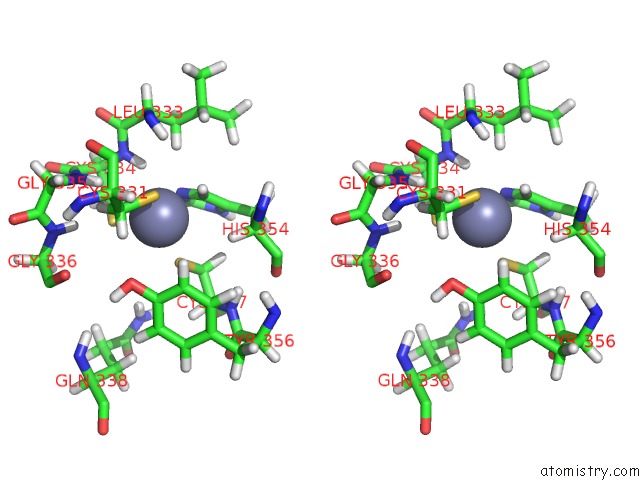
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of uc(Nmr) Structure of UHRF1 Phd Domains in A Complex with Histone H3 Peptide within 5.0Å range:
|
Zinc binding site 3 out of 3 in 2lgk
Go back to
Zinc binding site 3 out
of 3 in the uc(Nmr) Structure of UHRF1 Phd Domains in A Complex with Histone H3 Peptide
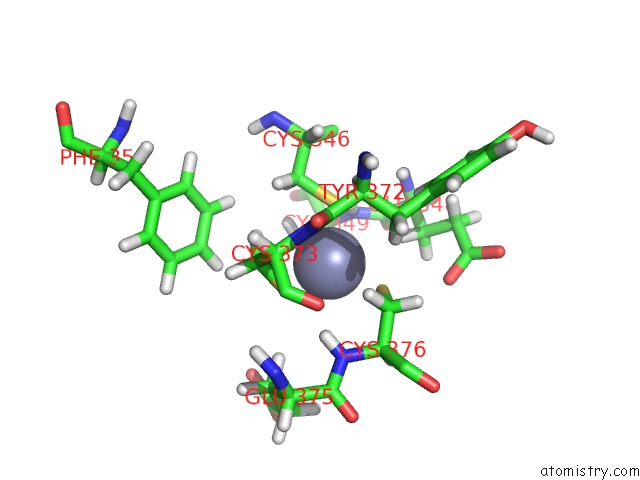
Mono view
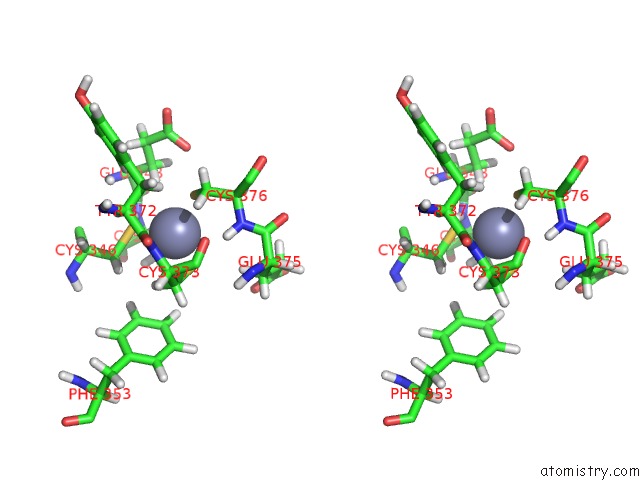
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 3 of uc(Nmr) Structure of UHRF1 Phd Domains in A Complex with Histone H3 Peptide within 5.0Å range:
|
Reference:
C.Wang,
J.Shen,
Z.Yang,
P.Chen,
B.Zhao,
W.Hu,
W.Lan,
X.Tong,
H.Wu,
G.Li,
C.Cao.
Structural Basis For Site-Specific Reading of Unmodified R2 of Histone H3 Tail By UHRF1 Phd Finger. Cell Res. V. 21 1379 2011.
ISSN: ISSN 1001-0602
PubMed: 21808299
DOI: 10.1038/CR.2011.123
Page generated: Thu Oct 17 01:50:35 2024
ISSN: ISSN 1001-0602
PubMed: 21808299
DOI: 10.1038/CR.2011.123
Last articles
Zn in 9JYWZn in 9IR4
Zn in 9IR3
Zn in 9GMX
Zn in 9GMW
Zn in 9JEJ
Zn in 9ERF
Zn in 9ERE
Zn in 9EGV
Zn in 9EGW