Zinc »
PDB 8c0q-8cd8 »
8c8u »
Zinc in PDB 8c8u: Priestia Megaterium Mupirocin-Resistant Isoleucyl-Trna Synthetase 2 Complexed with Mupirocin
Enzymatic activity of Priestia Megaterium Mupirocin-Resistant Isoleucyl-Trna Synthetase 2 Complexed with Mupirocin
All present enzymatic activity of Priestia Megaterium Mupirocin-Resistant Isoleucyl-Trna Synthetase 2 Complexed with Mupirocin:
6.1.1.5;
6.1.1.5;
Protein crystallography data
The structure of Priestia Megaterium Mupirocin-Resistant Isoleucyl-Trna Synthetase 2 Complexed with Mupirocin, PDB code: 8c8u
was solved by
A.Brkic,
M.Leibundgut,
J.Jablonska,
V.Zanki,
Z.Car,
V.Petrovic Perokovic,
I.Gruic-Sovulj,
N.Ban,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 48.23 / 1.90 |
| Space group | P 21 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 89.58, 124.83, 114.46, 90, 90, 90 |
| R / Rfree (%) | 18.3 / 22.2 |
Zinc Binding Sites:
The binding sites of Zinc atom in the Priestia Megaterium Mupirocin-Resistant Isoleucyl-Trna Synthetase 2 Complexed with Mupirocin
(pdb code 8c8u). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 2 binding sites of Zinc where determined in the Priestia Megaterium Mupirocin-Resistant Isoleucyl-Trna Synthetase 2 Complexed with Mupirocin, PDB code: 8c8u:
Jump to Zinc binding site number: 1; 2;
In total 2 binding sites of Zinc where determined in the Priestia Megaterium Mupirocin-Resistant Isoleucyl-Trna Synthetase 2 Complexed with Mupirocin, PDB code: 8c8u:
Jump to Zinc binding site number: 1; 2;
Zinc binding site 1 out of 2 in 8c8u
Go back to
Zinc binding site 1 out
of 2 in the Priestia Megaterium Mupirocin-Resistant Isoleucyl-Trna Synthetase 2 Complexed with Mupirocin
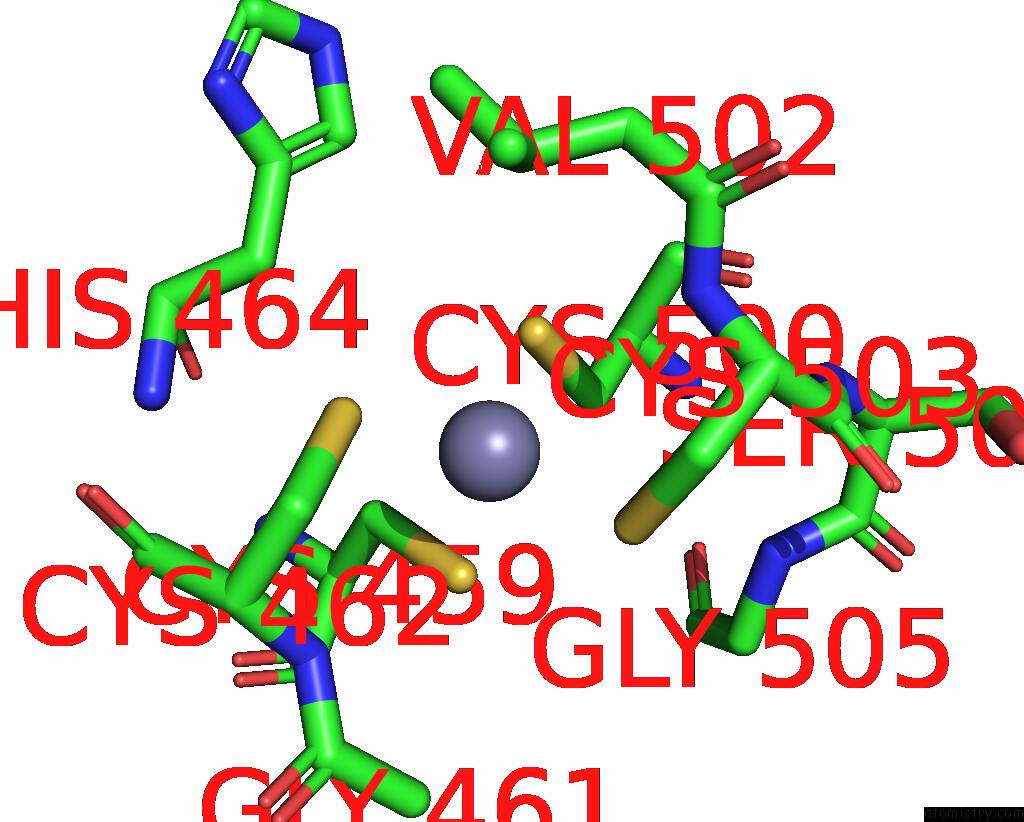
Mono view
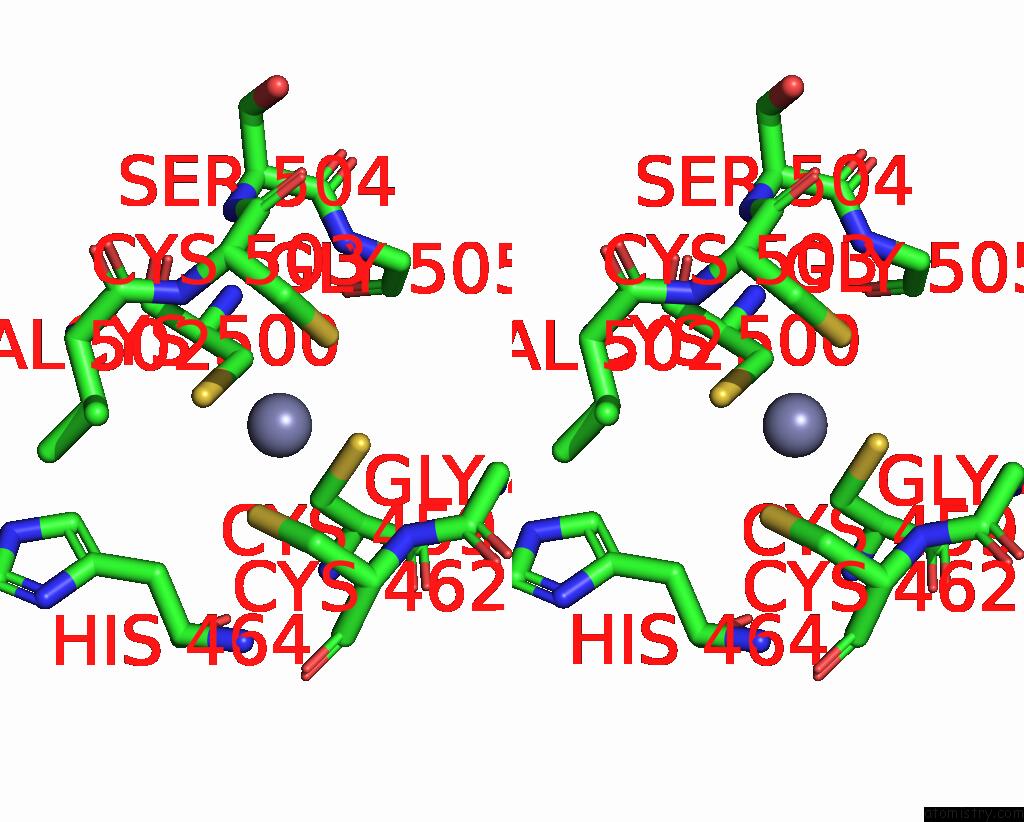
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Priestia Megaterium Mupirocin-Resistant Isoleucyl-Trna Synthetase 2 Complexed with Mupirocin within 5.0Å range:
|
Zinc binding site 2 out of 2 in 8c8u
Go back to
Zinc binding site 2 out
of 2 in the Priestia Megaterium Mupirocin-Resistant Isoleucyl-Trna Synthetase 2 Complexed with Mupirocin
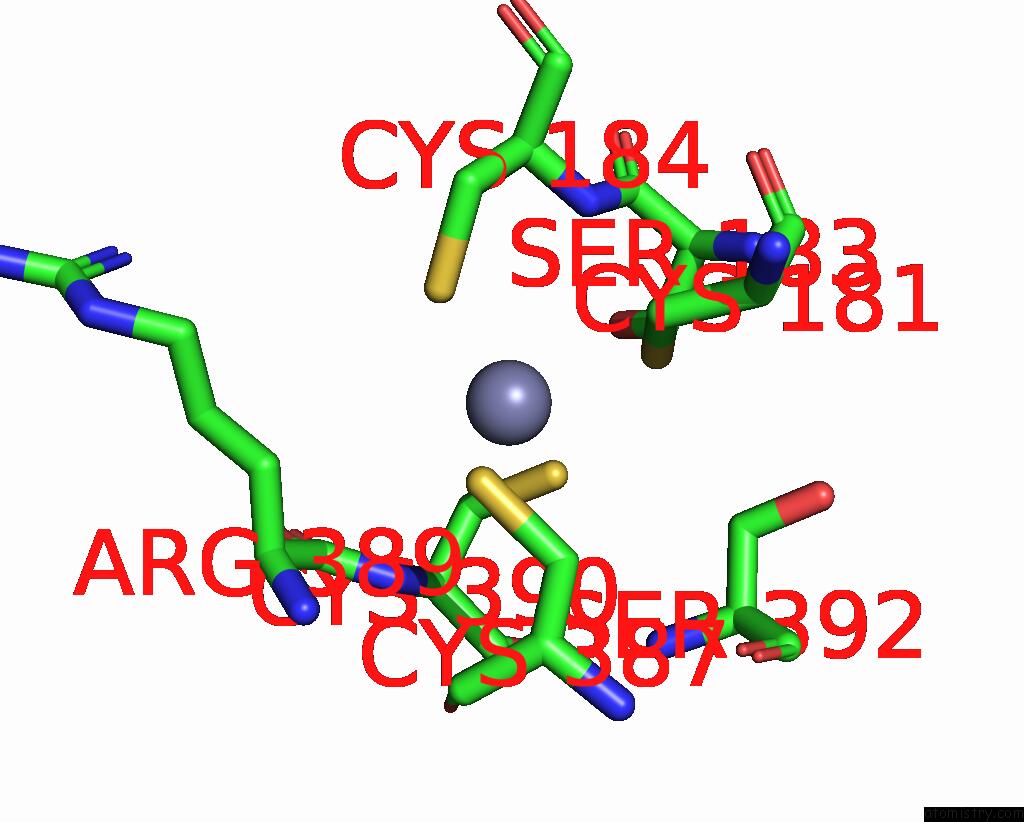
Mono view
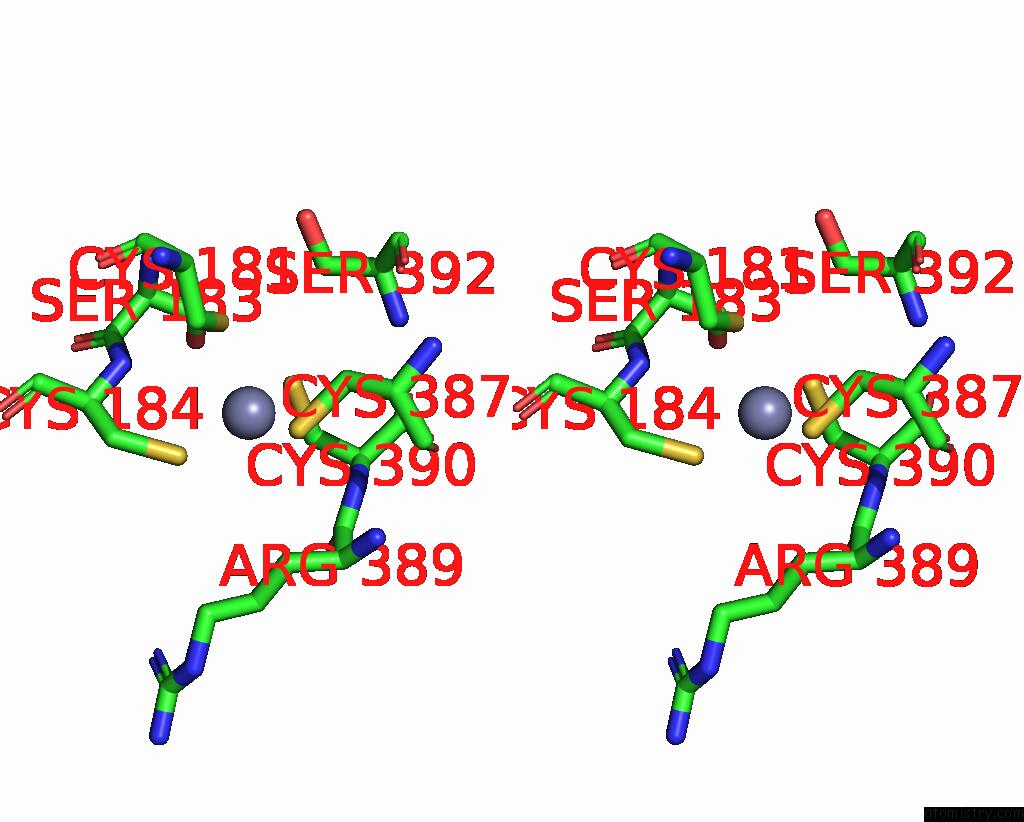
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of Priestia Megaterium Mupirocin-Resistant Isoleucyl-Trna Synthetase 2 Complexed with Mupirocin within 5.0Å range:
|
Reference:
A.Brkic,
M.Leibundgut,
J.Jablonska,
V.Zanki,
Z.Car,
V.Petrovic Perokovic,
N.Ban,
I.Gruic-Sovulj.
Antibiotic Hyper-Resistance in A Class I Aminoacyl-Trna Synthetase with Altered Active Site Signature Motif To Be Published.
Page generated: Fri Aug 22 08:51:03 2025
Last articles
Zn in 9BF8Zn in 9BDY
Zn in 9BCU
Zn in 9BCT
Zn in 9BDQ
Zn in 9B89
Zn in 9B4P
Zn in 9BAZ
Zn in 9BAQ
Zn in 9B85