Zinc »
PDB 8p7k-8pbg »
8pbe »
Zinc in PDB 8pbe: Mutant K1556T of the Dihydroorotase Domain of Human Cad Protein Bound to the Substrate Carbamoyl Aspartate
Enzymatic activity of Mutant K1556T of the Dihydroorotase Domain of Human Cad Protein Bound to the Substrate Carbamoyl Aspartate
All present enzymatic activity of Mutant K1556T of the Dihydroorotase Domain of Human Cad Protein Bound to the Substrate Carbamoyl Aspartate:
2.1.3.2; 3.5.2.3; 6.3.5.5;
2.1.3.2; 3.5.2.3; 6.3.5.5;
Protein crystallography data
The structure of Mutant K1556T of the Dihydroorotase Domain of Human Cad Protein Bound to the Substrate Carbamoyl Aspartate, PDB code: 8pbe
was solved by
F.Del Cano-Ochoa,
S.Ramon-Maiques,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 44.60 / 1.71 |
| Space group | C 2 2 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 82.189, 159.269, 61.857, 90, 90, 90 |
| R / Rfree (%) | 14.1 / 18.3 |
Zinc Binding Sites:
The binding sites of Zinc atom in the Mutant K1556T of the Dihydroorotase Domain of Human Cad Protein Bound to the Substrate Carbamoyl Aspartate
(pdb code 8pbe). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 2 binding sites of Zinc where determined in the Mutant K1556T of the Dihydroorotase Domain of Human Cad Protein Bound to the Substrate Carbamoyl Aspartate, PDB code: 8pbe:
Jump to Zinc binding site number: 1; 2;
In total 2 binding sites of Zinc where determined in the Mutant K1556T of the Dihydroorotase Domain of Human Cad Protein Bound to the Substrate Carbamoyl Aspartate, PDB code: 8pbe:
Jump to Zinc binding site number: 1; 2;
Zinc binding site 1 out of 2 in 8pbe
Go back to
Zinc binding site 1 out
of 2 in the Mutant K1556T of the Dihydroorotase Domain of Human Cad Protein Bound to the Substrate Carbamoyl Aspartate
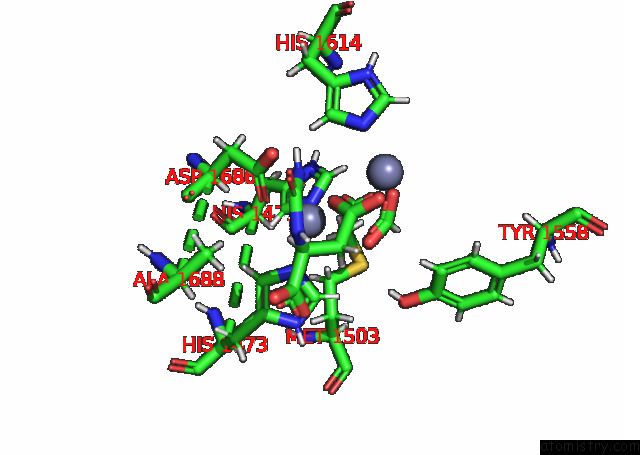
Mono view
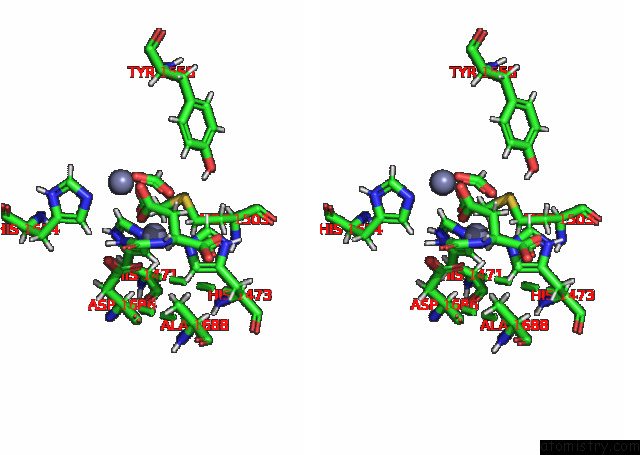
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Mutant K1556T of the Dihydroorotase Domain of Human Cad Protein Bound to the Substrate Carbamoyl Aspartate within 5.0Å range:
|
Zinc binding site 2 out of 2 in 8pbe
Go back to
Zinc binding site 2 out
of 2 in the Mutant K1556T of the Dihydroorotase Domain of Human Cad Protein Bound to the Substrate Carbamoyl Aspartate
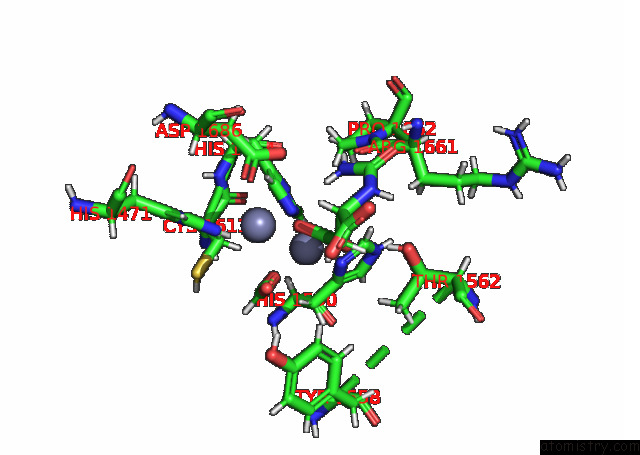
Mono view
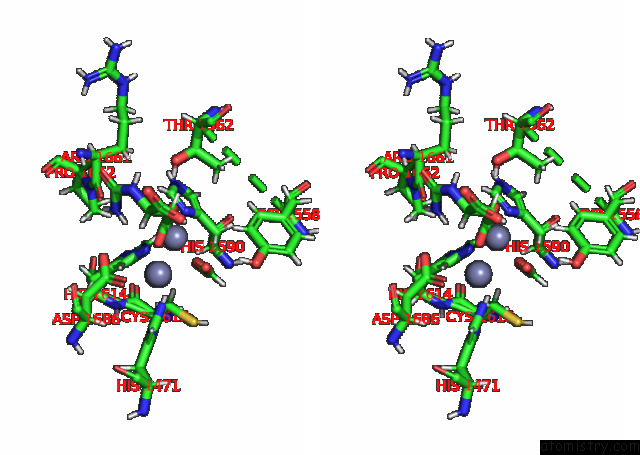
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of Mutant K1556T of the Dihydroorotase Domain of Human Cad Protein Bound to the Substrate Carbamoyl Aspartate within 5.0Å range:
|
Reference:
F.Del Cano-Ochoa,
B.G.Ng,
A.Rubio-Del-Campo,
S.Mahajan,
M.P.Wilson,
M.Vilar,
D.Rymen,
P.Sanchez-Pintos,
J.Kenny,
M.Ley Martos,
T.Campos,
S.B.Wortmann,
H.H.Freeze,
S.Ramon-Maiques.
Beyond Genetics: Deciphering the Impact of Missense Variants in Cad Deficiency. J Inherit Metab Dis 2023.
ISSN: ISSN 1573-2665
PubMed: 37540500
DOI: 10.1002/JIMD.12667
Page generated: Fri Aug 22 12:09:18 2025
ISSN: ISSN 1573-2665
PubMed: 37540500
DOI: 10.1002/JIMD.12667
Last articles
Zn in 9BCUZn in 9BCT
Zn in 9BDQ
Zn in 9B89
Zn in 9B4P
Zn in 9BAZ
Zn in 9BAQ
Zn in 9B85
Zn in 9BAP
Zn in 9B84