Zinc »
PDB 8ccz-8cx2 »
8cg7 »
Zinc in PDB 8cg7: Structure of P53 Cancer Mutant Y220C with Arylation at CYS182 and CYS277
Protein crystallography data
The structure of Structure of P53 Cancer Mutant Y220C with Arylation at CYS182 and CYS277, PDB code: 8cg7
was solved by
D.I.Balourdas,
M.M.Pichon,
M.G.J.Baud,
S.Knapp,
A.C.Joerger,
Structuralgenomics Consortium (Sgc),
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 47.90 / 1.53 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 64.881, 71.046, 104.887, 90, 90, 90 |
| R / Rfree (%) | 15.8 / 18.7 |
Other elements in 8cg7:
The structure of Structure of P53 Cancer Mutant Y220C with Arylation at CYS182 and CYS277 also contains other interesting chemical elements:
| Iodine | (I) | 4 atoms |
Zinc Binding Sites:
The binding sites of Zinc atom in the Structure of P53 Cancer Mutant Y220C with Arylation at CYS182 and CYS277
(pdb code 8cg7). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 2 binding sites of Zinc where determined in the Structure of P53 Cancer Mutant Y220C with Arylation at CYS182 and CYS277, PDB code: 8cg7:
Jump to Zinc binding site number: 1; 2;
In total 2 binding sites of Zinc where determined in the Structure of P53 Cancer Mutant Y220C with Arylation at CYS182 and CYS277, PDB code: 8cg7:
Jump to Zinc binding site number: 1; 2;
Zinc binding site 1 out of 2 in 8cg7
Go back to
Zinc binding site 1 out
of 2 in the Structure of P53 Cancer Mutant Y220C with Arylation at CYS182 and CYS277
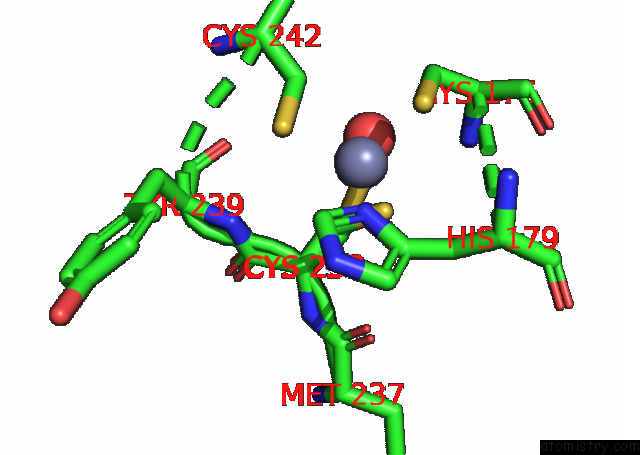
Mono view
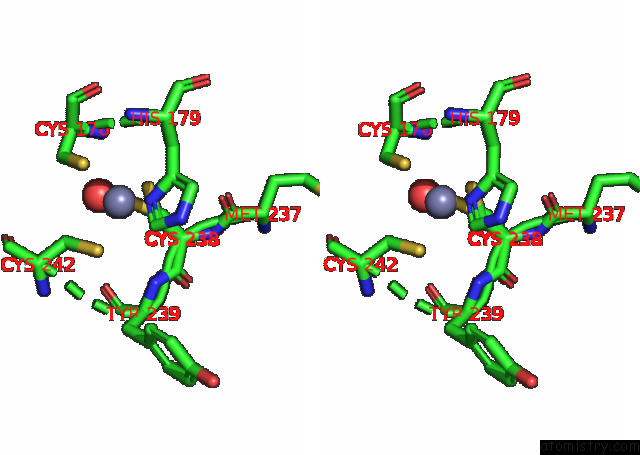
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Structure of P53 Cancer Mutant Y220C with Arylation at CYS182 and CYS277 within 5.0Å range:
|
Zinc binding site 2 out of 2 in 8cg7
Go back to
Zinc binding site 2 out
of 2 in the Structure of P53 Cancer Mutant Y220C with Arylation at CYS182 and CYS277
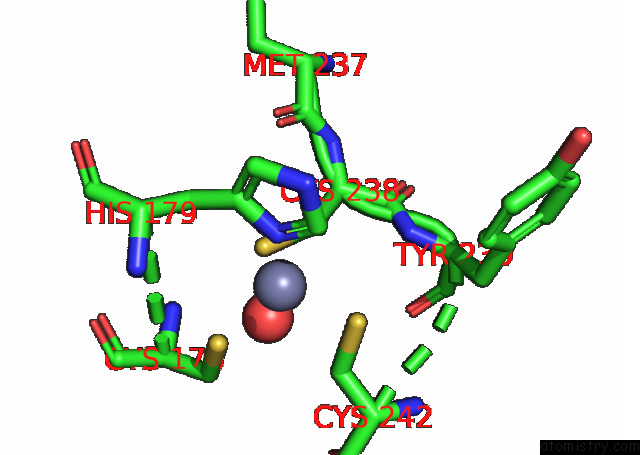
Mono view
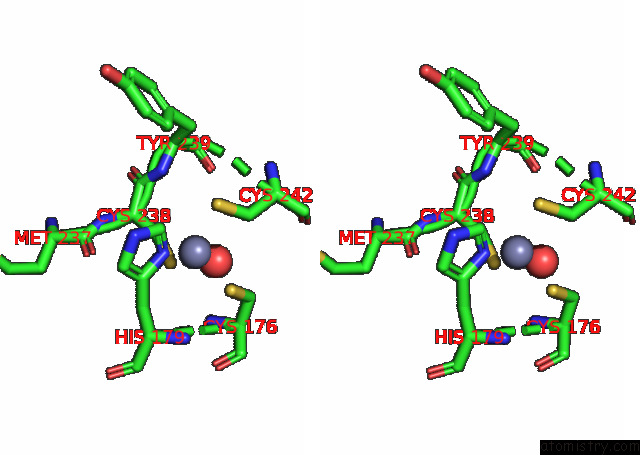
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of Structure of P53 Cancer Mutant Y220C with Arylation at CYS182 and CYS277 within 5.0Å range:
|
Reference:
M.M.Pichon,
D.Drelinkiewicz,
D.Lozano,
R.Moraru,
L.J.Hayward,
M.Jones,
M.A.Mccoy,
S.Allstrum-Graves,
D.I.Balourdas,
A.C.Joerger,
R.J.Whitby,
S.M.Goldup,
N.Wells,
G.J.Langley,
J.M.Herniman,
M.G.J.Baud.
Structure-Reactivity Studies of 2-Sulfonylpyrimidines Allow Selective Protein Arylation. Bioconjug.Chem. V. 34 1679 2023.
ISSN: ISSN 1043-1802
PubMed: 37657082
DOI: 10.1021/ACS.BIOCONJCHEM.3C00322
Page generated: Thu Dec 28 12:56:11 2023
ISSN: ISSN 1043-1802
PubMed: 37657082
DOI: 10.1021/ACS.BIOCONJCHEM.3C00322
Last articles
Zn in 8WB0Zn in 8WAX
Zn in 8WAU
Zn in 8WAZ
Zn in 8WAY
Zn in 8WAV
Zn in 8WAW
Zn in 8WAT
Zn in 8W7M
Zn in 8WD3