Zinc »
PDB 1y8q-1ykf »
1yaz »
Zinc in PDB 1yaz: Azide-Bound Yeast Cu(II)/Zn Superoxide Dismutase Room Temperature (298K) Structure
Enzymatic activity of Azide-Bound Yeast Cu(II)/Zn Superoxide Dismutase Room Temperature (298K) Structure
All present enzymatic activity of Azide-Bound Yeast Cu(II)/Zn Superoxide Dismutase Room Temperature (298K) Structure:
1.15.1.1;
1.15.1.1;
Protein crystallography data
The structure of Azide-Bound Yeast Cu(II)/Zn Superoxide Dismutase Room Temperature (298K) Structure, PDB code: 1yaz
was solved by
P.J.Hart,
M.M.Balbirnie,
N.L.Ogihara,
A.M.Nersissian,
M.S.Weiss,
J.S.Valentine,
D.Eisenberg,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 90.00 / 1.70 |
| Space group | H 3 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 119.375, 119.375, 74.543, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 16.9 / 20.8 |
Other elements in 1yaz:
The structure of Azide-Bound Yeast Cu(II)/Zn Superoxide Dismutase Room Temperature (298K) Structure also contains other interesting chemical elements:
| Copper | (Cu) | 1 atom |
Zinc Binding Sites:
The binding sites of Zinc atom in the Azide-Bound Yeast Cu(II)/Zn Superoxide Dismutase Room Temperature (298K) Structure
(pdb code 1yaz). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total only one binding site of Zinc was determined in the Azide-Bound Yeast Cu(II)/Zn Superoxide Dismutase Room Temperature (298K) Structure, PDB code: 1yaz:
In total only one binding site of Zinc was determined in the Azide-Bound Yeast Cu(II)/Zn Superoxide Dismutase Room Temperature (298K) Structure, PDB code: 1yaz:
Zinc binding site 1 out of 1 in 1yaz
Go back to
Zinc binding site 1 out
of 1 in the Azide-Bound Yeast Cu(II)/Zn Superoxide Dismutase Room Temperature (298K) Structure

Mono view
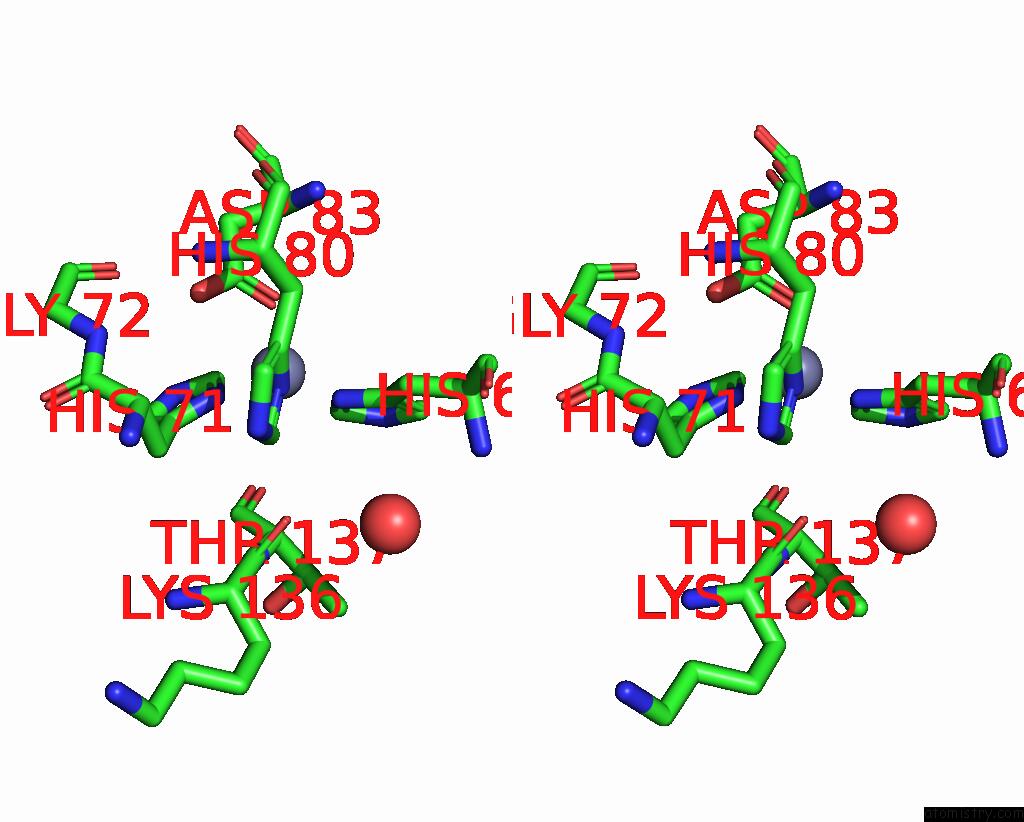
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Azide-Bound Yeast Cu(II)/Zn Superoxide Dismutase Room Temperature (298K) Structure within 5.0Å range:
|
Reference:
P.J.Hart,
M.M.Balbirnie,
N.L.Ogihara,
A.M.Nersissian,
M.S.Weiss,
J.S.Valentine,
D.Eisenberg.
A Structure-Based Mechanism For Copper-Zinc Superoxide Dismutase. Biochemistry V. 38 2167 1999.
ISSN: ISSN 0006-2960
PubMed: 10026301
DOI: 10.1021/BI982284U
Page generated: Wed Aug 20 00:34:46 2025
ISSN: ISSN 0006-2960
PubMed: 10026301
DOI: 10.1021/BI982284U
Last articles
Zn in 2MUMZn in 2MUR
Zn in 2MUQ
Zn in 2MS0
Zn in 2MS1
Zn in 2MS3
Zn in 2MQ1
Zn in 2MRF
Zn in 2MQV
Zn in 2MRE