Zinc »
PDB 6eea-6eox »
6enm »
Zinc in PDB 6enm: Crystal Structure of MMP12 in Complex with Hydroxamate Inhibitor LP168.
Enzymatic activity of Crystal Structure of MMP12 in Complex with Hydroxamate Inhibitor LP168.
All present enzymatic activity of Crystal Structure of MMP12 in Complex with Hydroxamate Inhibitor LP168.:
3.4.24.65;
3.4.24.65;
Protein crystallography data
The structure of Crystal Structure of MMP12 in Complex with Hydroxamate Inhibitor LP168., PDB code: 6enm
was solved by
L.Vera,
E.Nuti,
A.Rossello,
E.A.Stura,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 47.91 / 1.59 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 61.410, 67.090, 68.440, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 17.4 / 20.2 |
Other elements in 6enm:
The structure of Crystal Structure of MMP12 in Complex with Hydroxamate Inhibitor LP168. also contains other interesting chemical elements:
| Calcium | (Ca) | 6 atoms |
Zinc Binding Sites:
The binding sites of Zinc atom in the Crystal Structure of MMP12 in Complex with Hydroxamate Inhibitor LP168.
(pdb code 6enm). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 4 binding sites of Zinc where determined in the Crystal Structure of MMP12 in Complex with Hydroxamate Inhibitor LP168., PDB code: 6enm:
Jump to Zinc binding site number: 1; 2; 3; 4;
In total 4 binding sites of Zinc where determined in the Crystal Structure of MMP12 in Complex with Hydroxamate Inhibitor LP168., PDB code: 6enm:
Jump to Zinc binding site number: 1; 2; 3; 4;
Zinc binding site 1 out of 4 in 6enm
Go back to
Zinc binding site 1 out
of 4 in the Crystal Structure of MMP12 in Complex with Hydroxamate Inhibitor LP168.
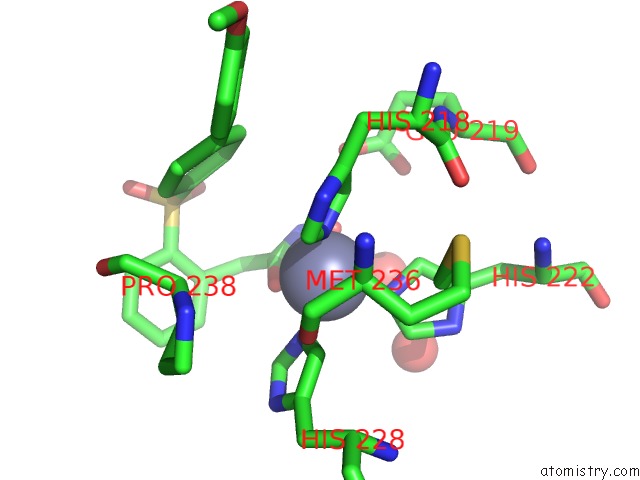
Mono view
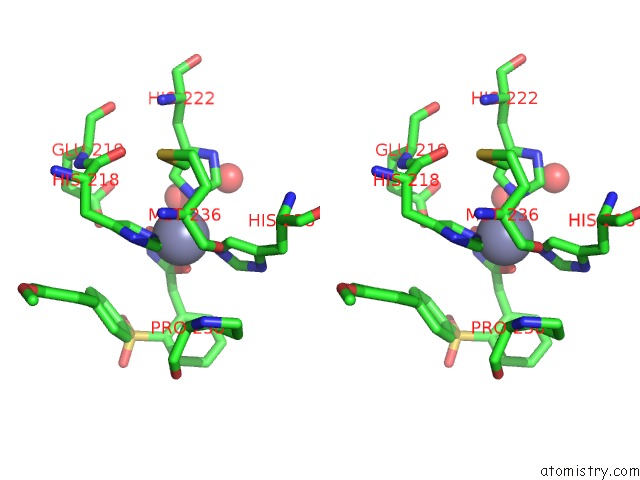
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Crystal Structure of MMP12 in Complex with Hydroxamate Inhibitor LP168. within 5.0Å range:
|
Zinc binding site 2 out of 4 in 6enm
Go back to
Zinc binding site 2 out
of 4 in the Crystal Structure of MMP12 in Complex with Hydroxamate Inhibitor LP168.
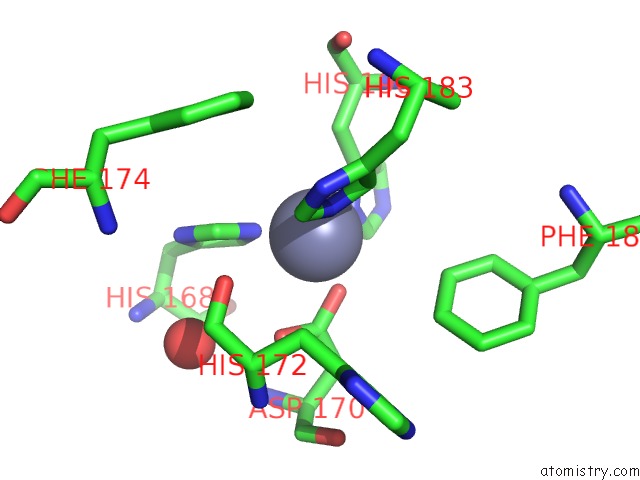
Mono view
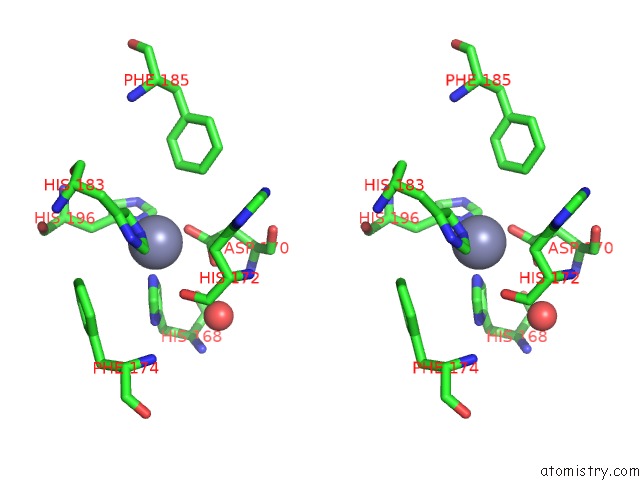
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of Crystal Structure of MMP12 in Complex with Hydroxamate Inhibitor LP168. within 5.0Å range:
|
Zinc binding site 3 out of 4 in 6enm
Go back to
Zinc binding site 3 out
of 4 in the Crystal Structure of MMP12 in Complex with Hydroxamate Inhibitor LP168.
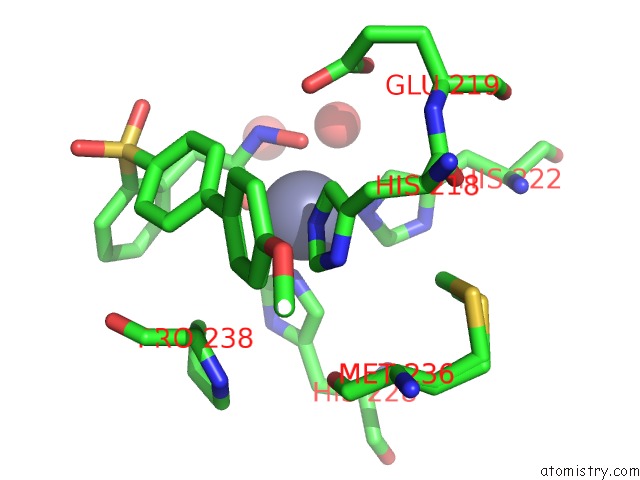
Mono view
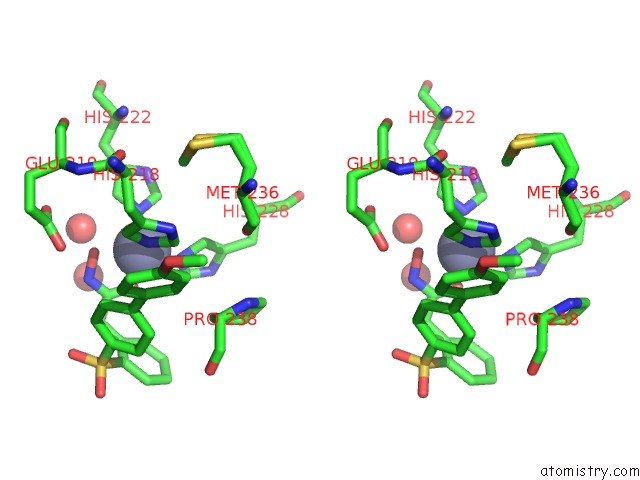
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 3 of Crystal Structure of MMP12 in Complex with Hydroxamate Inhibitor LP168. within 5.0Å range:
|
Zinc binding site 4 out of 4 in 6enm
Go back to
Zinc binding site 4 out
of 4 in the Crystal Structure of MMP12 in Complex with Hydroxamate Inhibitor LP168.
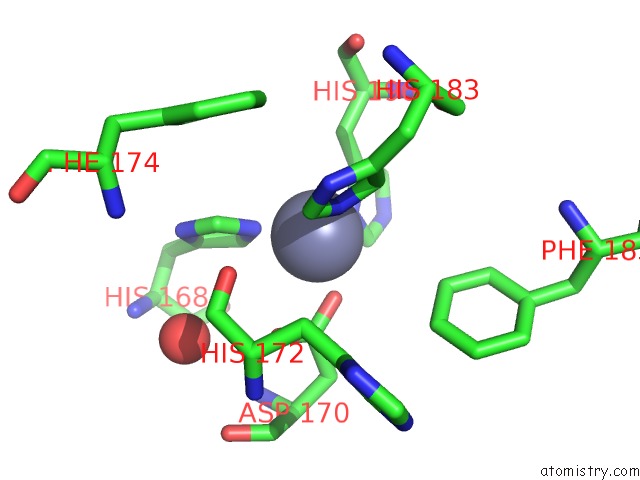
Mono view
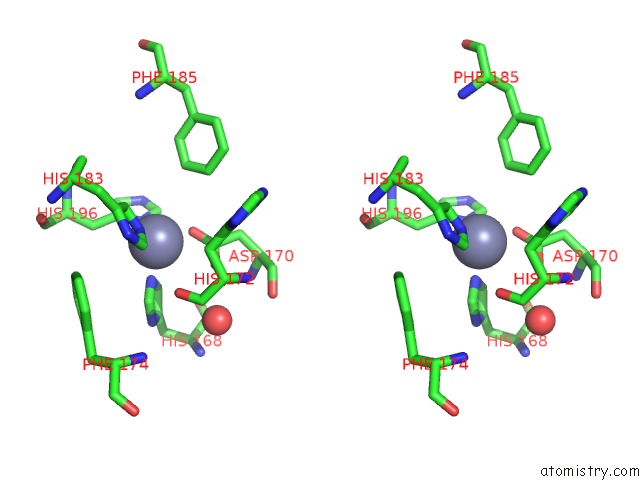
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 4 of Crystal Structure of MMP12 in Complex with Hydroxamate Inhibitor LP168. within 5.0Å range:
|
Reference:
E.Nuti,
D.Cuffaro,
E.Bernardini,
C.Camodeca,
L.Panelli,
S.Chaves,
L.Ciccone,
L.Tepshi,
L.Vera,
E.Orlandini,
S.Nencetti,
E.A.Stura,
M.A.Santos,
V.Dive,
A.Rossello.
Development of Thioaryl-Based Matrix Metalloproteinase-12 Inhibitors with Alternative Zinc-Binding Groups: Synthesis, Potentiometric, uc(Nmr), and Crystallographic Studies. J. Med. Chem. V. 61 4421 2018.
ISSN: ISSN 1520-4804
PubMed: 29727184
DOI: 10.1021/ACS.JMEDCHEM.8B00096
Page generated: Mon Oct 28 20:16:12 2024
ISSN: ISSN 1520-4804
PubMed: 29727184
DOI: 10.1021/ACS.JMEDCHEM.8B00096
Last articles
Zn in 9J0NZn in 9J0O
Zn in 9J0P
Zn in 9FJX
Zn in 9EKB
Zn in 9C0F
Zn in 9CAH
Zn in 9CH0
Zn in 9CH3
Zn in 9CH1