Zinc »
PDB 6btn-6c6u »
6c06 »
Zinc in PDB 6c06: Mycobacterium Tuberculosis Rnap Holo/Rbpa/Fidaxomicin
Enzymatic activity of Mycobacterium Tuberculosis Rnap Holo/Rbpa/Fidaxomicin
All present enzymatic activity of Mycobacterium Tuberculosis Rnap Holo/Rbpa/Fidaxomicin:
2.7.7.6;
2.7.7.6;
Other elements in 6c06:
The structure of Mycobacterium Tuberculosis Rnap Holo/Rbpa/Fidaxomicin also contains other interesting chemical elements:
| Magnesium | (Mg) | 1 atom |
| Chlorine | (Cl) | 2 atoms |
Zinc Binding Sites:
The binding sites of Zinc atom in the Mycobacterium Tuberculosis Rnap Holo/Rbpa/Fidaxomicin
(pdb code 6c06). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 2 binding sites of Zinc where determined in the Mycobacterium Tuberculosis Rnap Holo/Rbpa/Fidaxomicin, PDB code: 6c06:
Jump to Zinc binding site number: 1; 2;
In total 2 binding sites of Zinc where determined in the Mycobacterium Tuberculosis Rnap Holo/Rbpa/Fidaxomicin, PDB code: 6c06:
Jump to Zinc binding site number: 1; 2;
Zinc binding site 1 out of 2 in 6c06
Go back to
Zinc binding site 1 out
of 2 in the Mycobacterium Tuberculosis Rnap Holo/Rbpa/Fidaxomicin
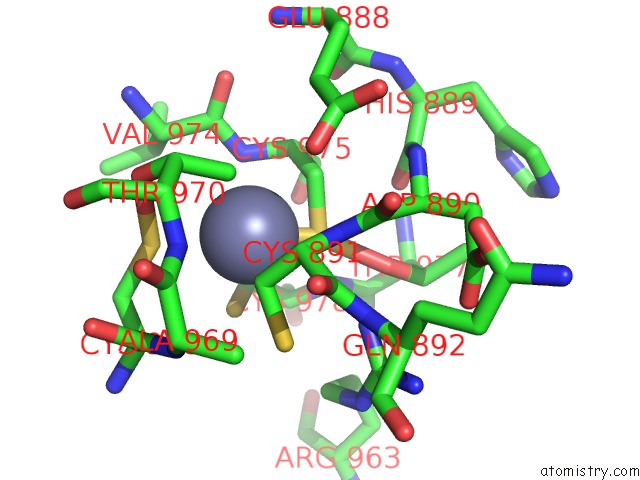
Mono view
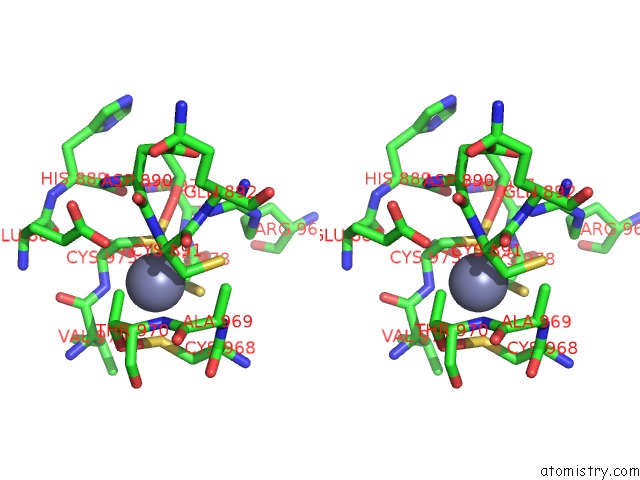
Stereo pair view

Mono view

Stereo pair view
|
|
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Mycobacterium Tuberculosis Rnap Holo/Rbpa/Fidaxomicin within 5.0Å range:
|
Zinc binding site 2 out of 2 in 6c06
Go back to
Zinc binding site 2 out
of 2 in the Mycobacterium Tuberculosis Rnap Holo/Rbpa/Fidaxomicin
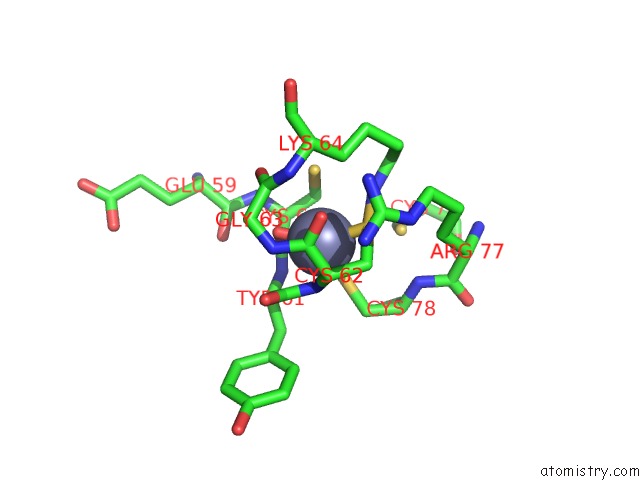
Mono view
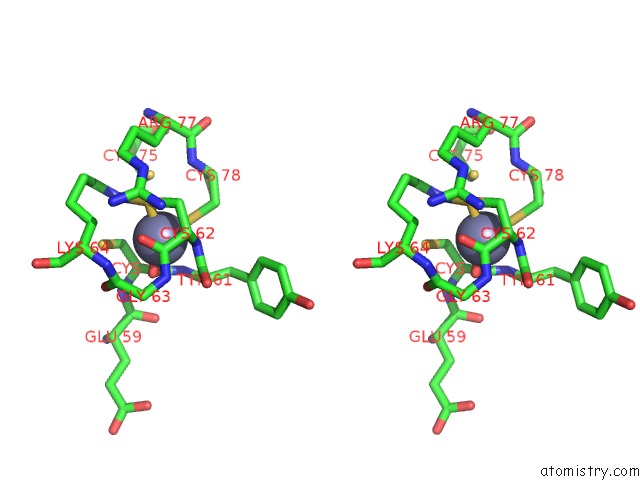
Stereo pair view

Mono view

Stereo pair view
|
|
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of Mycobacterium Tuberculosis Rnap Holo/Rbpa/Fidaxomicin within 5.0Å range:
|
Reference:
H.Boyaci,
J.Chen,
M.Lilic,
M.Palka,
R.A.Mooney,
R.Landick,
S.A.Darst,
E.A.Campbell.
Fidaxomicin Jamsmycobacterium Tuberculosisrna Polymerase Motions Needed For Initiation Via Rbpa Contacts. Elife V. 7 2018.
ISSN: ESSN 2050-084X
PubMed: 29480804
DOI: 10.7554/ELIFE.34823
Page generated: Mon Oct 28 18:25:33 2024
ISSN: ESSN 2050-084X
PubMed: 29480804
DOI: 10.7554/ELIFE.34823
Last articles
Zn in 9J0NZn in 9J0O
Zn in 9J0P
Zn in 9FJX
Zn in 9EKB
Zn in 9C0F
Zn in 9CAH
Zn in 9CH0
Zn in 9CH3
Zn in 9CH1