Zinc »
PDB 3sjf-3suf »
3suf »
Zinc in PDB 3suf: Crystal Structure of NS3/4A Protease Variant D168A in Complex with Mk- 5172
Protein crystallography data
The structure of Crystal Structure of NS3/4A Protease Variant D168A in Complex with Mk- 5172, PDB code: 3suf
was solved by
C.A.Schiffer,
K.P.Romano,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 29.22 / 2.19 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 56.004, 103.560, 73.510, 90.00, 112.04, 90.00 |
| R / Rfree (%) | 19.9 / 25.9 |
Zinc Binding Sites:
The binding sites of Zinc atom in the Crystal Structure of NS3/4A Protease Variant D168A in Complex with Mk- 5172
(pdb code 3suf). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 4 binding sites of Zinc where determined in the Crystal Structure of NS3/4A Protease Variant D168A in Complex with Mk- 5172, PDB code: 3suf:
Jump to Zinc binding site number: 1; 2; 3; 4;
In total 4 binding sites of Zinc where determined in the Crystal Structure of NS3/4A Protease Variant D168A in Complex with Mk- 5172, PDB code: 3suf:
Jump to Zinc binding site number: 1; 2; 3; 4;
Zinc binding site 1 out of 4 in 3suf
Go back to
Zinc binding site 1 out
of 4 in the Crystal Structure of NS3/4A Protease Variant D168A in Complex with Mk- 5172
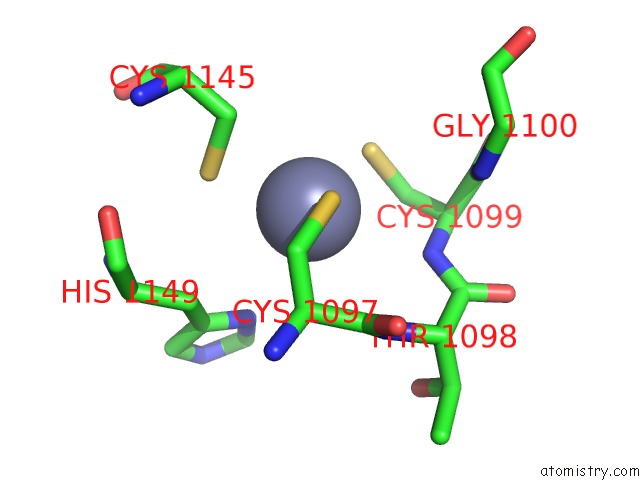
Mono view
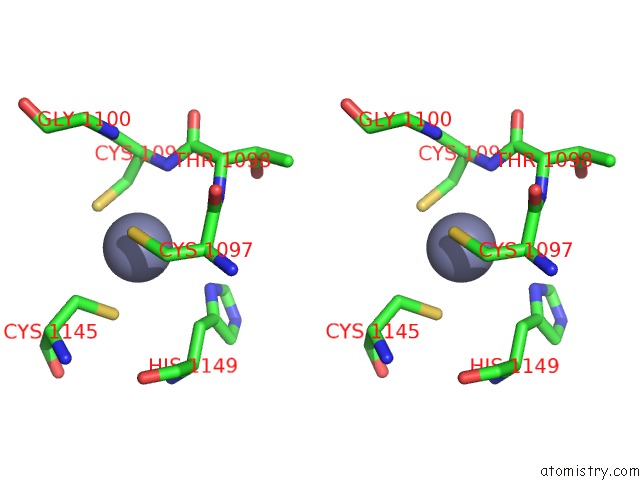
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Crystal Structure of NS3/4A Protease Variant D168A in Complex with Mk- 5172 within 5.0Å range:
|
Zinc binding site 2 out of 4 in 3suf
Go back to
Zinc binding site 2 out
of 4 in the Crystal Structure of NS3/4A Protease Variant D168A in Complex with Mk- 5172
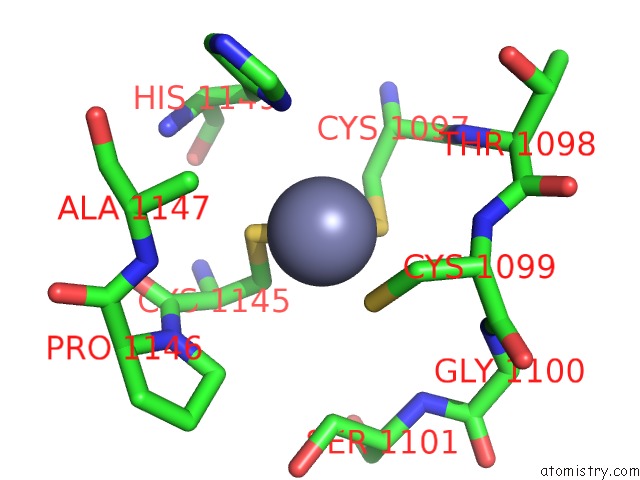
Mono view
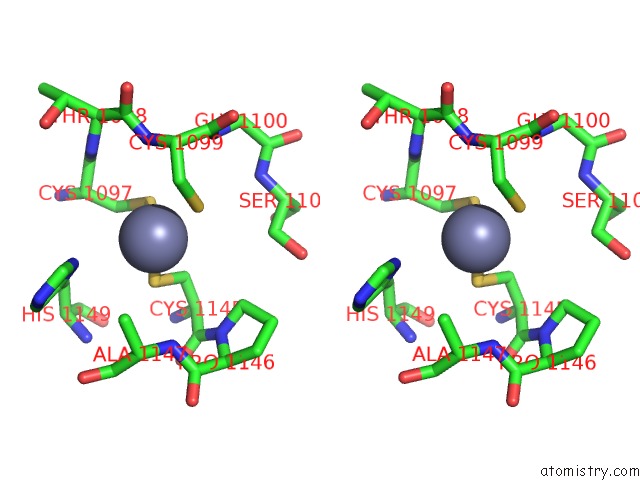
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of Crystal Structure of NS3/4A Protease Variant D168A in Complex with Mk- 5172 within 5.0Å range:
|
Zinc binding site 3 out of 4 in 3suf
Go back to
Zinc binding site 3 out
of 4 in the Crystal Structure of NS3/4A Protease Variant D168A in Complex with Mk- 5172
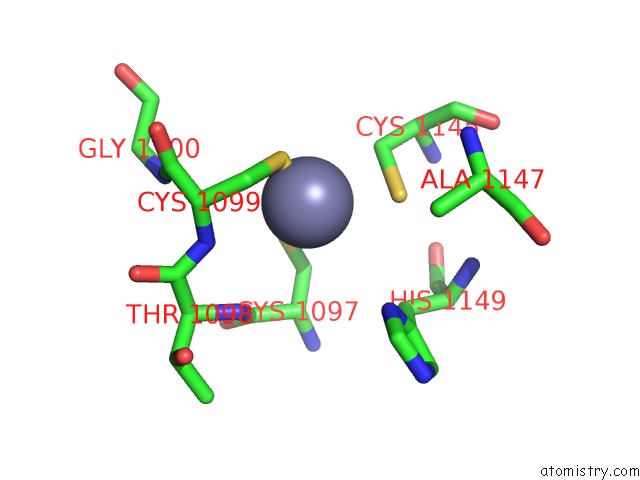
Mono view
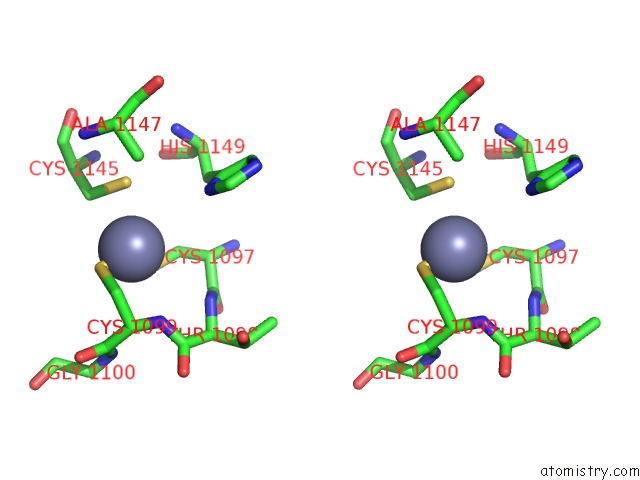
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 3 of Crystal Structure of NS3/4A Protease Variant D168A in Complex with Mk- 5172 within 5.0Å range:
|
Zinc binding site 4 out of 4 in 3suf
Go back to
Zinc binding site 4 out
of 4 in the Crystal Structure of NS3/4A Protease Variant D168A in Complex with Mk- 5172
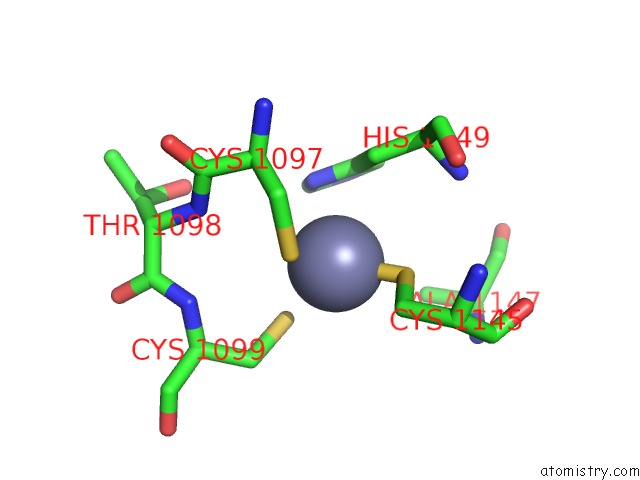
Mono view
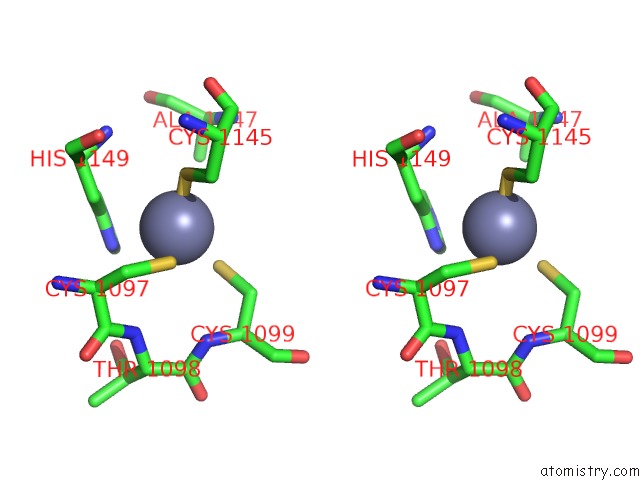
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 4 of Crystal Structure of NS3/4A Protease Variant D168A in Complex with Mk- 5172 within 5.0Å range:
|
Reference:
K.P.Romano,
A.Ali,
C.Aydin,
D.Soumana,
A.Ozen,
L.M.Deveau,
C.Silver,
H.Cao,
A.Newton,
C.J.Petropoulos,
W.Huang,
C.A.Schiffer.
The Molecular Basis of Drug Resistance Against Hepatitis C Virus NS3/4A Protease Inhibitors. Plos Pathog. V. 8 02832 2012.
ISSN: ISSN 1553-7366
PubMed: 22910833
DOI: 10.1371/JOURNAL.PPAT.1002832
Page generated: Wed Aug 20 14:13:51 2025
ISSN: ISSN 1553-7366
PubMed: 22910833
DOI: 10.1371/JOURNAL.PPAT.1002832
Last articles
Zn in 4KYHZn in 4KXQ
Zn in 4KXD
Zn in 4KXB
Zn in 4KXC
Zn in 4KXA
Zn in 4KW9
Zn in 4KVP
Zn in 4KX9
Zn in 4KX8