Zinc »
PDB 2ow0-2pcu »
2ow2 »
Zinc in PDB 2ow2: Mmp-9 Active Site Mutant with Difluoro Butanoic Acid Inhibitor
Enzymatic activity of Mmp-9 Active Site Mutant with Difluoro Butanoic Acid Inhibitor
All present enzymatic activity of Mmp-9 Active Site Mutant with Difluoro Butanoic Acid Inhibitor:
3.4.24.35;
3.4.24.35;
Protein crystallography data
The structure of Mmp-9 Active Site Mutant with Difluoro Butanoic Acid Inhibitor, PDB code: 2ow2
was solved by
A.Tochowicz,
W.Bode,
K.Maskos,
P.Goettig,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 19.73 / 2.90 |
| Space group | P 41 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 55.420, 55.420, 260.793, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 21.5 / 28.5 |
Other elements in 2ow2:
The structure of Mmp-9 Active Site Mutant with Difluoro Butanoic Acid Inhibitor also contains other interesting chemical elements:
| Fluorine | (F) | 4 atoms |
| Chlorine | (Cl) | 2 atoms |
| Calcium | (Ca) | 6 atoms |
Zinc Binding Sites:
The binding sites of Zinc atom in the Mmp-9 Active Site Mutant with Difluoro Butanoic Acid Inhibitor
(pdb code 2ow2). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 4 binding sites of Zinc where determined in the Mmp-9 Active Site Mutant with Difluoro Butanoic Acid Inhibitor, PDB code: 2ow2:
Jump to Zinc binding site number: 1; 2; 3; 4;
In total 4 binding sites of Zinc where determined in the Mmp-9 Active Site Mutant with Difluoro Butanoic Acid Inhibitor, PDB code: 2ow2:
Jump to Zinc binding site number: 1; 2; 3; 4;
Zinc binding site 1 out of 4 in 2ow2
Go back to
Zinc binding site 1 out
of 4 in the Mmp-9 Active Site Mutant with Difluoro Butanoic Acid Inhibitor
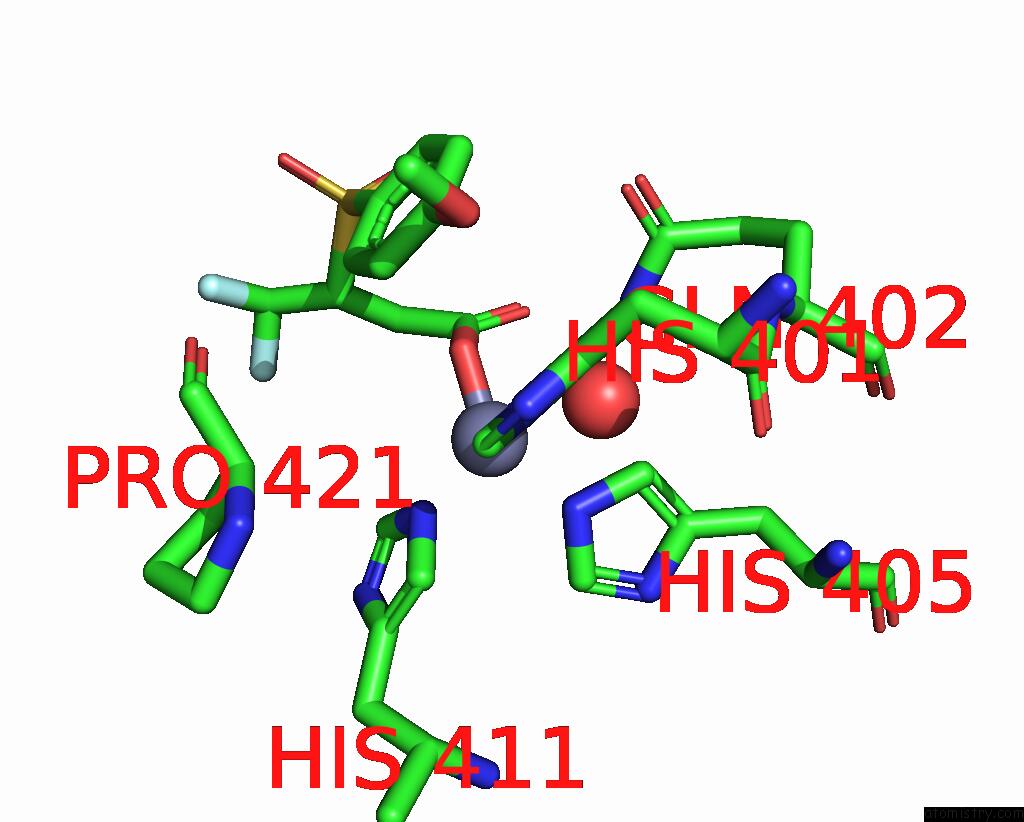
Mono view
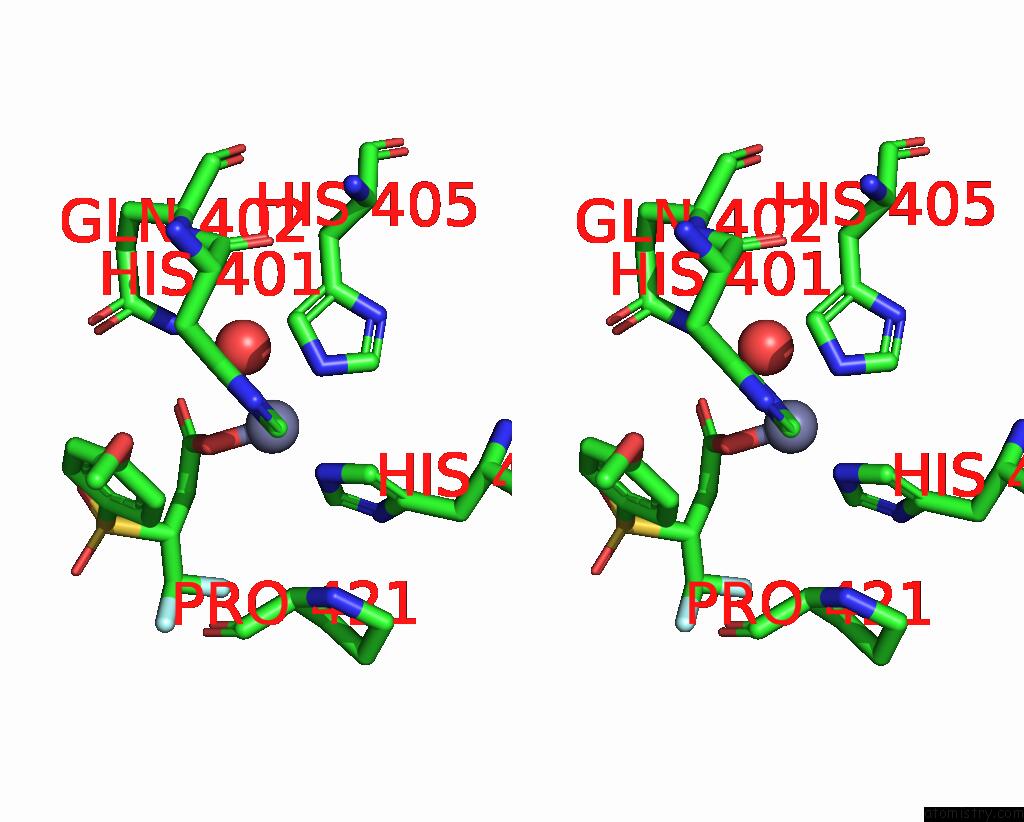
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Mmp-9 Active Site Mutant with Difluoro Butanoic Acid Inhibitor within 5.0Å range:
|
Zinc binding site 2 out of 4 in 2ow2
Go back to
Zinc binding site 2 out
of 4 in the Mmp-9 Active Site Mutant with Difluoro Butanoic Acid Inhibitor
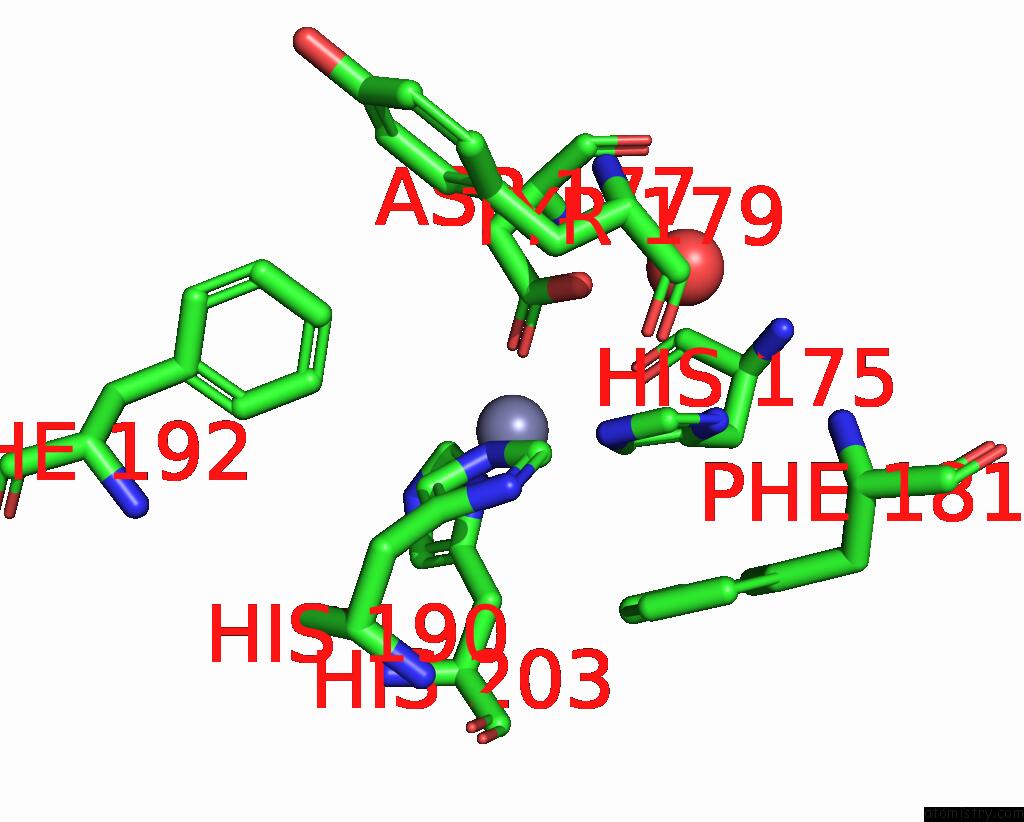
Mono view
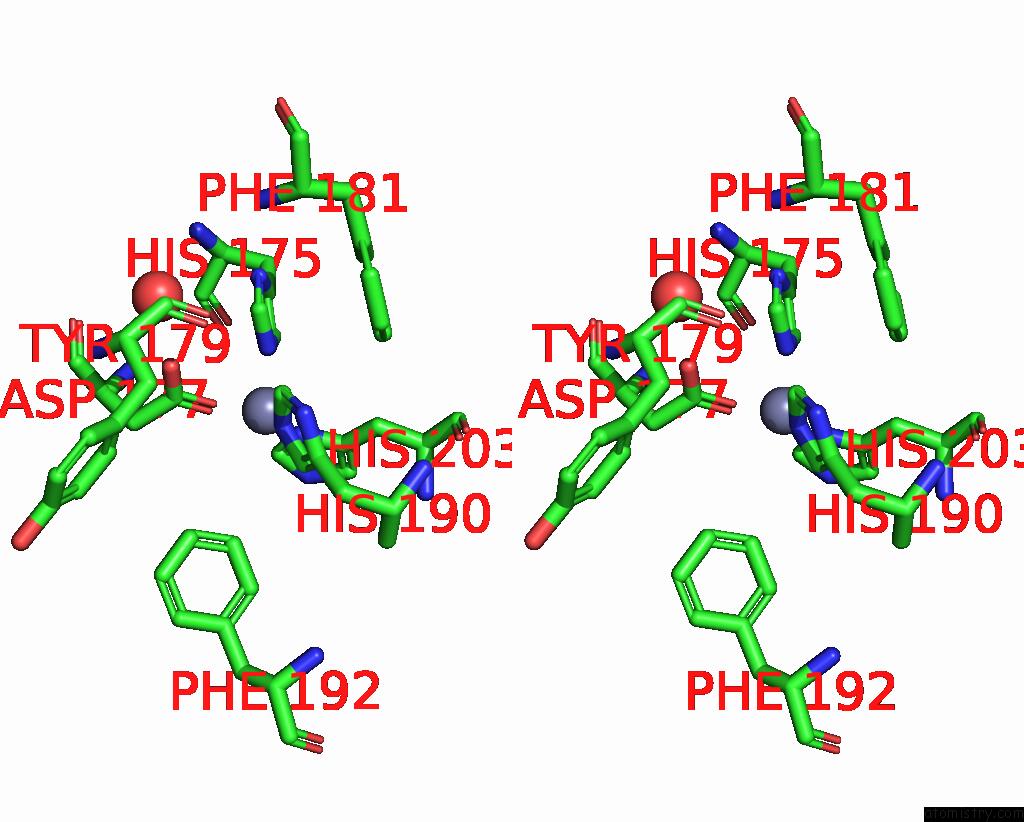
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of Mmp-9 Active Site Mutant with Difluoro Butanoic Acid Inhibitor within 5.0Å range:
|
Zinc binding site 3 out of 4 in 2ow2
Go back to
Zinc binding site 3 out
of 4 in the Mmp-9 Active Site Mutant with Difluoro Butanoic Acid Inhibitor
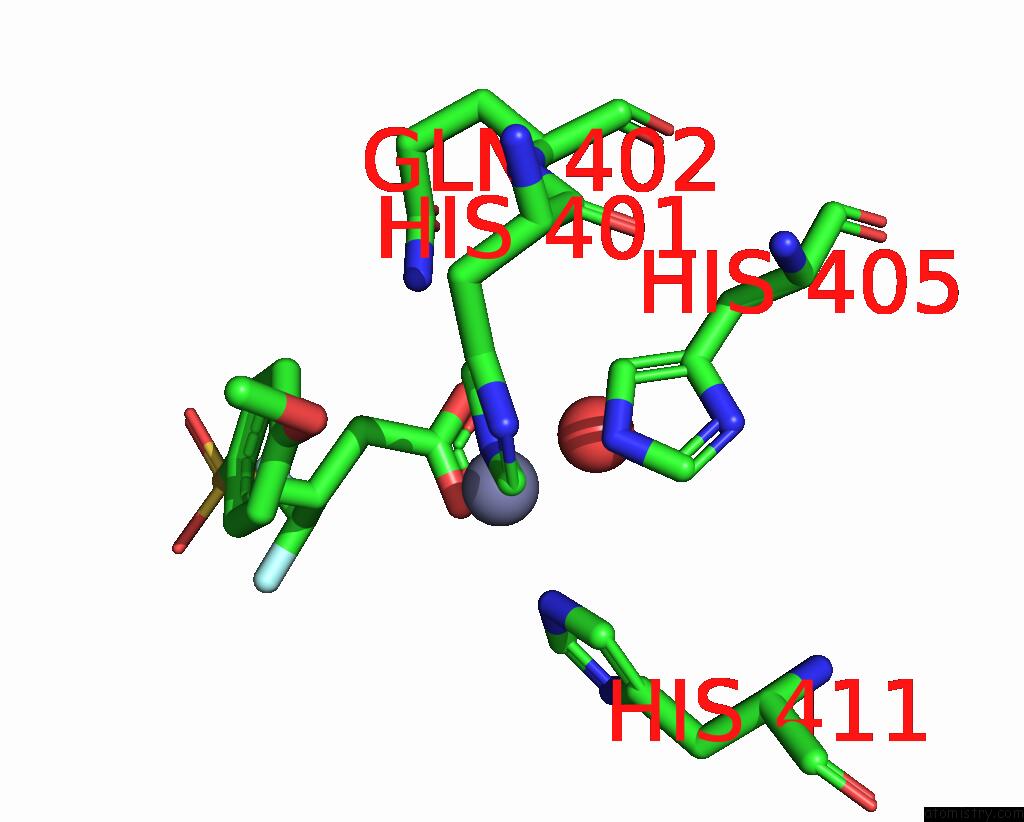
Mono view
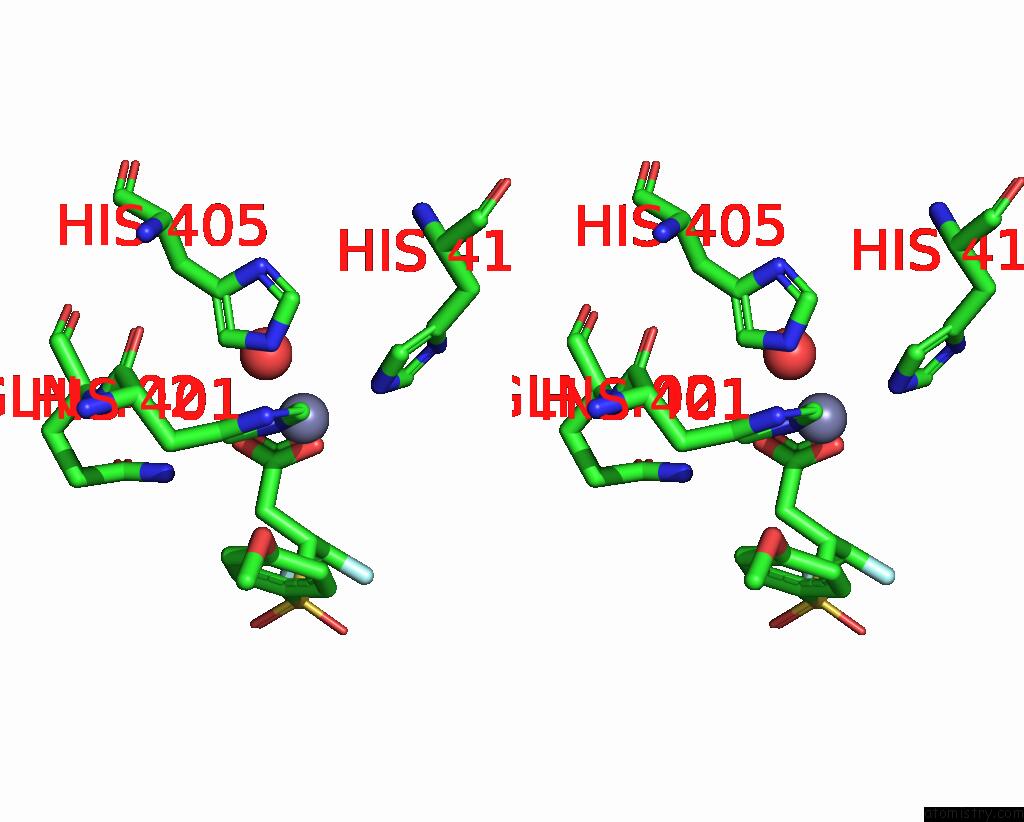
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 3 of Mmp-9 Active Site Mutant with Difluoro Butanoic Acid Inhibitor within 5.0Å range:
|
Zinc binding site 4 out of 4 in 2ow2
Go back to
Zinc binding site 4 out
of 4 in the Mmp-9 Active Site Mutant with Difluoro Butanoic Acid Inhibitor
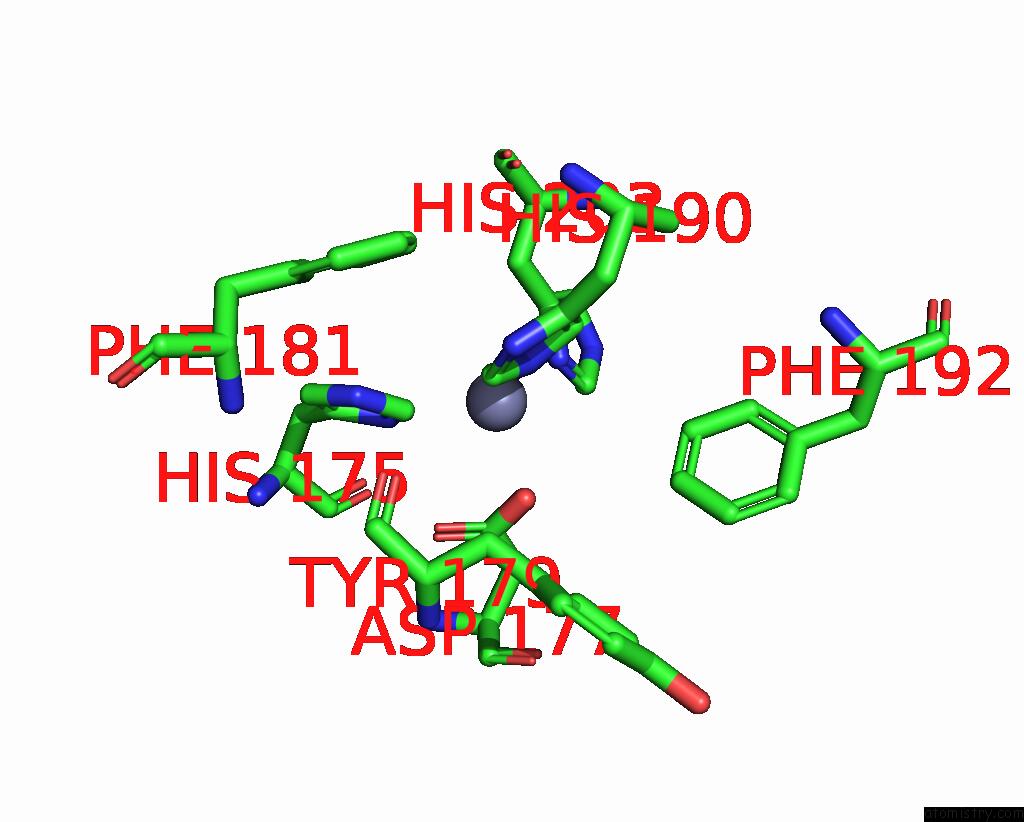
Mono view
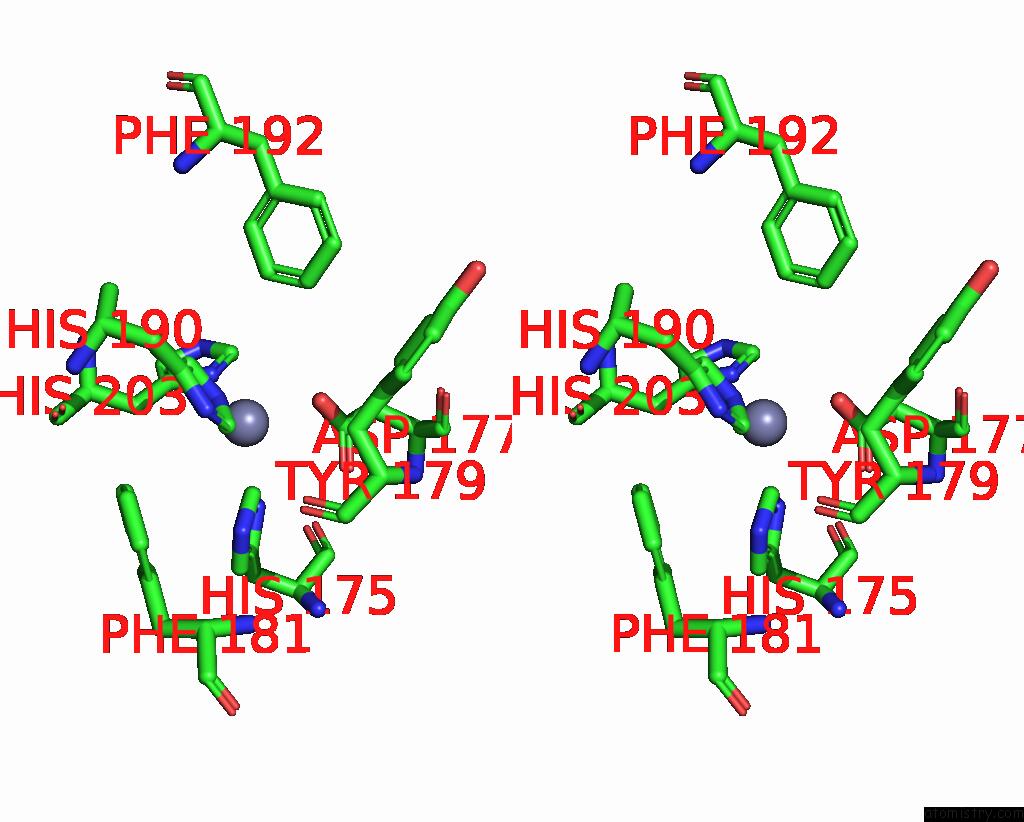
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 4 of Mmp-9 Active Site Mutant with Difluoro Butanoic Acid Inhibitor within 5.0Å range:
|
Reference:
A.Tochowicz,
K.Maskos,
R.Huber,
R.Oltenfreiter,
V.Dive,
A.Yiotakis,
M.Zanda,
W.Bode,
P.Goettig.
Crystal Structures of Mmp-9 Complexes with Five Inhibitors: Contribution of the Flexible ARG424 Side-Chain to Selectivity. J.Mol.Biol. V. 371 989 2007.
ISSN: ISSN 0022-2836
PubMed: 17599356
DOI: 10.1016/J.JMB.2007.05.068
Page generated: Thu Oct 17 02:49:13 2024
ISSN: ISSN 0022-2836
PubMed: 17599356
DOI: 10.1016/J.JMB.2007.05.068
Last articles
Zn in 9J0NZn in 9J0O
Zn in 9J0P
Zn in 9FJX
Zn in 9EKB
Zn in 9C0F
Zn in 9CAH
Zn in 9CH0
Zn in 9CH3
Zn in 9CH1