Zinc »
PDB 2mmi-2n9p »
2n1a »
Zinc in PDB 2n1a: Docked Structure Between SUMO1 and Zz-Domain From Cbp
Enzymatic activity of Docked Structure Between SUMO1 and Zz-Domain From Cbp
All present enzymatic activity of Docked Structure Between SUMO1 and Zz-Domain From Cbp:
2.3.1.48;
2.3.1.48;
Zinc Binding Sites:
The binding sites of Zinc atom in the Docked Structure Between SUMO1 and Zz-Domain From Cbp
(pdb code 2n1a). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 2 binding sites of Zinc where determined in the Docked Structure Between SUMO1 and Zz-Domain From Cbp, PDB code: 2n1a:
Jump to Zinc binding site number: 1; 2;
In total 2 binding sites of Zinc where determined in the Docked Structure Between SUMO1 and Zz-Domain From Cbp, PDB code: 2n1a:
Jump to Zinc binding site number: 1; 2;
Zinc binding site 1 out of 2 in 2n1a
Go back to
Zinc binding site 1 out
of 2 in the Docked Structure Between SUMO1 and Zz-Domain From Cbp
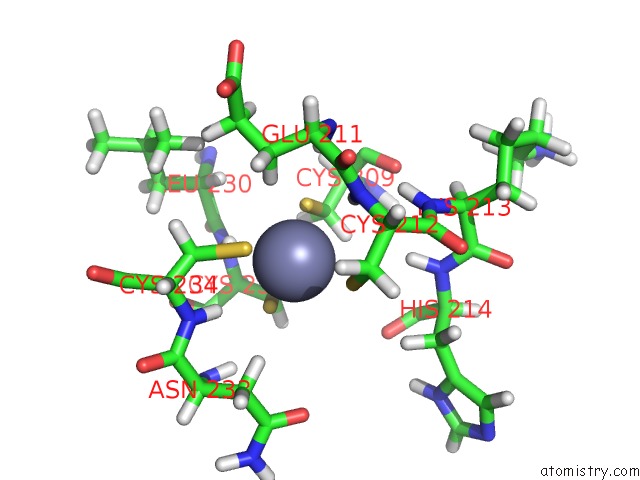
Mono view
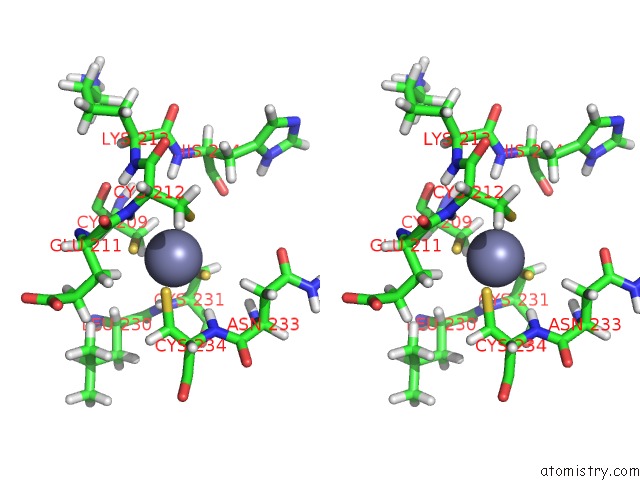
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Docked Structure Between SUMO1 and Zz-Domain From Cbp within 5.0Å range:
|
Zinc binding site 2 out of 2 in 2n1a
Go back to
Zinc binding site 2 out
of 2 in the Docked Structure Between SUMO1 and Zz-Domain From Cbp
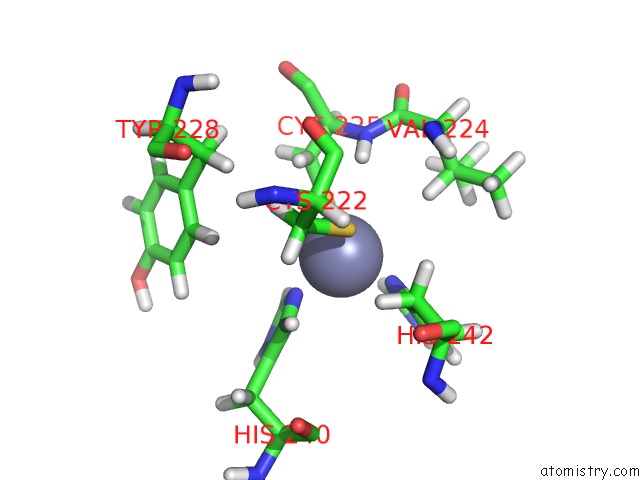
Mono view
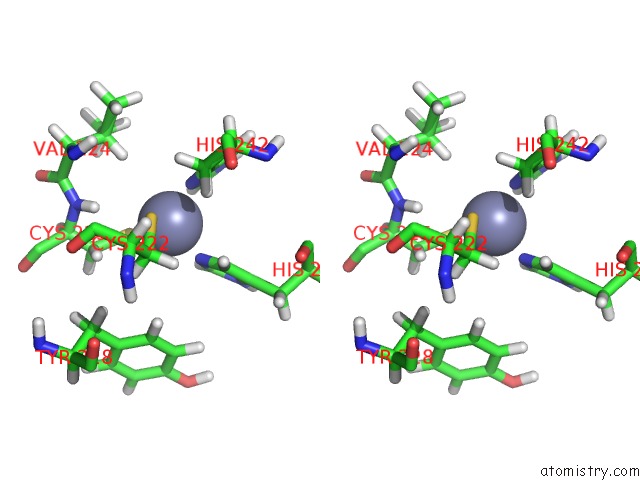
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of Docked Structure Between SUMO1 and Zz-Domain From Cbp within 5.0Å range:
|
Reference:
C.Diehl,
M.Akke,
S.Bekker-Jensen,
N.Mailand,
W.Streicher,
M.Wikstrom.
Structural Analysis of A Complex Between Small Ubiquitin-Like Modifier 1 (SUMO1) and the Zz Domain of Creb-Binding Protein (Cbp/P300) Reveals A New Interaction Surface on Sumo. J.Biol.Chem. V. 291 12658 2016.
ISSN: ISSN 0021-9258
PubMed: 27129204
DOI: 10.1074/JBC.M115.711325
Page generated: Thu Oct 17 02:11:05 2024
ISSN: ISSN 0021-9258
PubMed: 27129204
DOI: 10.1074/JBC.M115.711325
Last articles
Zn in 9J0NZn in 9J0O
Zn in 9J0P
Zn in 9FJX
Zn in 9EKB
Zn in 9C0F
Zn in 9CAH
Zn in 9CH0
Zn in 9CH3
Zn in 9CH1