Zinc »
PDB 2kfn-2kzy »
2kyu »
Zinc in PDB 2kyu: The Solution Structure of the PHD3 Finger of Mll
Zinc Binding Sites:
The binding sites of Zinc atom in the The Solution Structure of the PHD3 Finger of Mll
(pdb code 2kyu). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 2 binding sites of Zinc where determined in the The Solution Structure of the PHD3 Finger of Mll, PDB code: 2kyu:
Jump to Zinc binding site number: 1; 2;
In total 2 binding sites of Zinc where determined in the The Solution Structure of the PHD3 Finger of Mll, PDB code: 2kyu:
Jump to Zinc binding site number: 1; 2;
Zinc binding site 1 out of 2 in 2kyu
Go back to
Zinc binding site 1 out
of 2 in the The Solution Structure of the PHD3 Finger of Mll
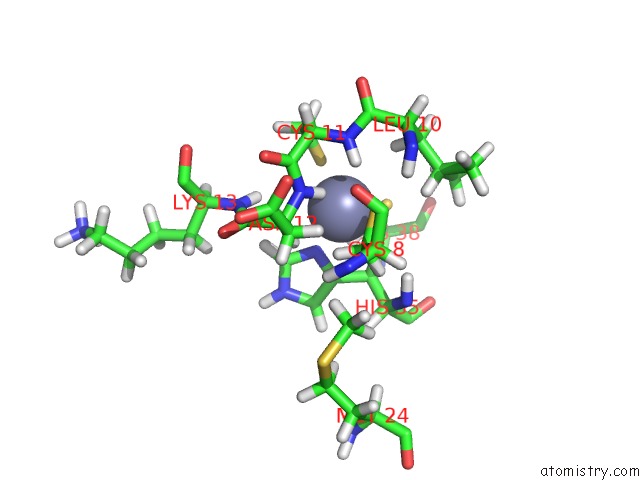
Mono view
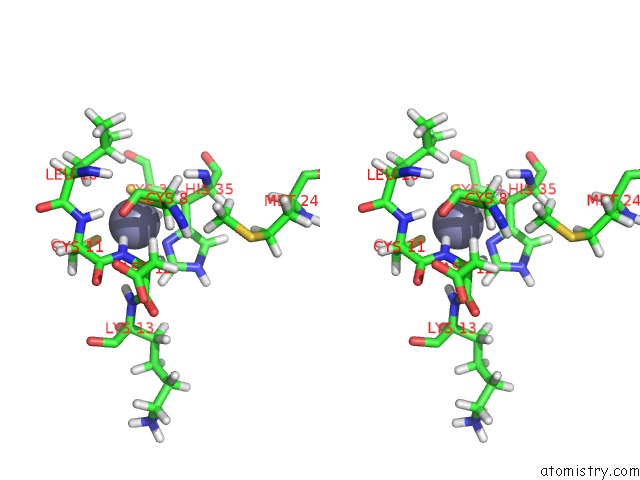
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of The Solution Structure of the PHD3 Finger of Mll within 5.0Å range:
|
Zinc binding site 2 out of 2 in 2kyu
Go back to
Zinc binding site 2 out
of 2 in the The Solution Structure of the PHD3 Finger of Mll
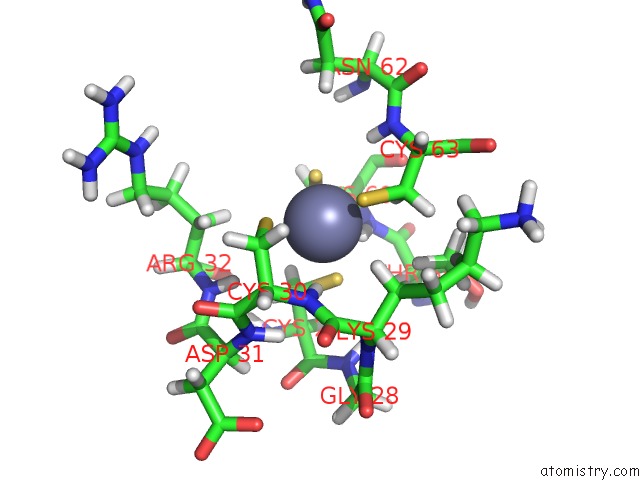
Mono view
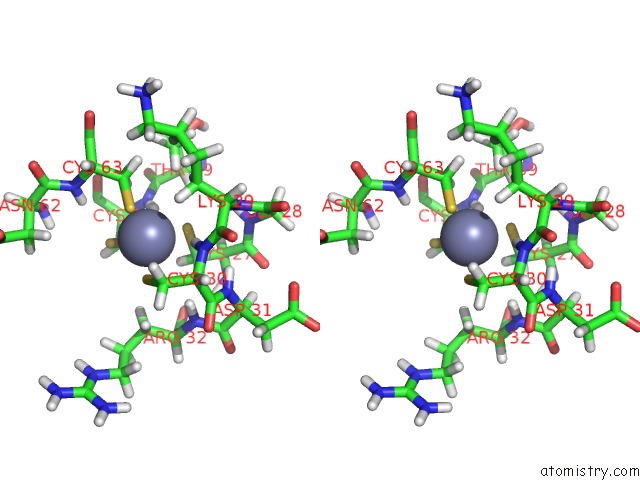
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of The Solution Structure of the PHD3 Finger of Mll within 5.0Å range:
|
Reference:
S.Park,
U.Osmers,
G.Raman,
R.H.Schwantes,
M.O.Diaz,
J.H.Bushweller.
The PHD3 Domain of Mll Acts As A CYP33-Regulated Switch Between Mll-Mediated Activation and Repression. Biochemistry V. 49 6576 2010.
ISSN: ISSN 0006-2960
PubMed: 20677832
DOI: 10.1021/BI1009387
Page generated: Thu Oct 17 01:42:41 2024
ISSN: ISSN 0006-2960
PubMed: 20677832
DOI: 10.1021/BI1009387
Last articles
Zn in 9J0NZn in 9J0O
Zn in 9J0P
Zn in 9FJX
Zn in 9EKB
Zn in 9C0F
Zn in 9CAH
Zn in 9CH0
Zn in 9CH3
Zn in 9CH1