Zinc »
PDB 2kfn-2kzy »
2kwo »
Zinc in PDB 2kwo: Solution Structure of the Double Phd (Plant Homeodomain) Fingers of Human Transcriptional Protein DPF3B Bound to A Histone H4 Peptide Containing N-Terminal Acetylation at Serine 1
Zinc Binding Sites:
The binding sites of Zinc atom in the Solution Structure of the Double Phd (Plant Homeodomain) Fingers of Human Transcriptional Protein DPF3B Bound to A Histone H4 Peptide Containing N-Terminal Acetylation at Serine 1
(pdb code 2kwo). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 4 binding sites of Zinc where determined in the Solution Structure of the Double Phd (Plant Homeodomain) Fingers of Human Transcriptional Protein DPF3B Bound to A Histone H4 Peptide Containing N-Terminal Acetylation at Serine 1, PDB code: 2kwo:
Jump to Zinc binding site number: 1; 2; 3; 4;
In total 4 binding sites of Zinc where determined in the Solution Structure of the Double Phd (Plant Homeodomain) Fingers of Human Transcriptional Protein DPF3B Bound to A Histone H4 Peptide Containing N-Terminal Acetylation at Serine 1, PDB code: 2kwo:
Jump to Zinc binding site number: 1; 2; 3; 4;
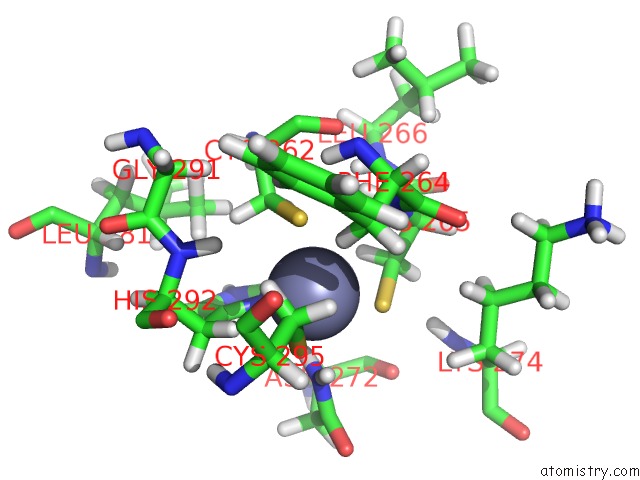
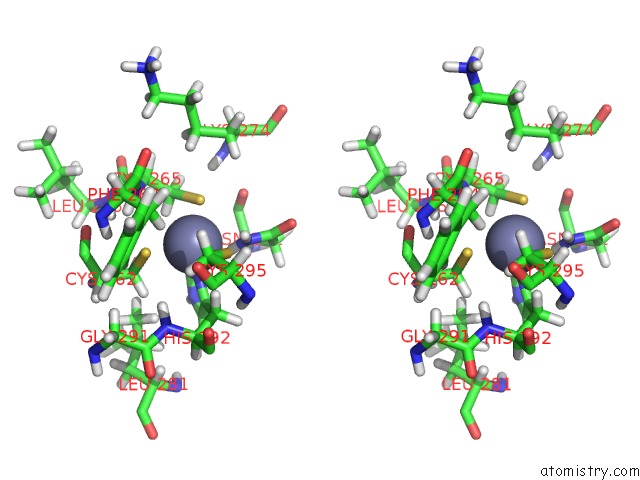
Zinc binding site 1 out of 4 in 2kwo
Go back to
Zinc binding site 1 out
of 4 in the Solution Structure of the Double Phd (Plant Homeodomain) Fingers of Human Transcriptional Protein DPF3B Bound to A Histone H4 Peptide Containing N-Terminal Acetylation at Serine 1
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Solution Structure of the Double Phd (Plant Homeodomain) Fingers of Human Transcriptional Protein DPF3B Bound to A Histone H4 Peptide Containing N-Terminal Acetylation at Serine 1 within 5.0Å range:
|
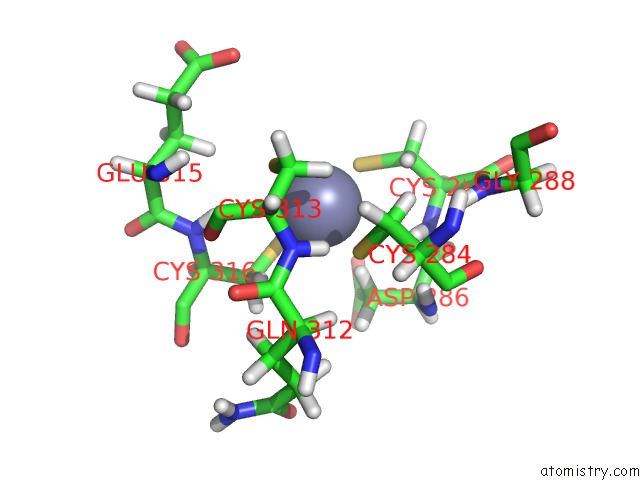
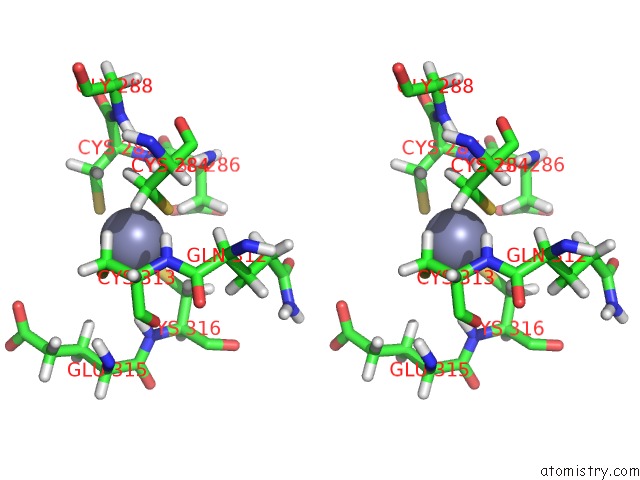
Zinc binding site 2 out of 4 in 2kwo
Go back to
Zinc binding site 2 out
of 4 in the Solution Structure of the Double Phd (Plant Homeodomain) Fingers of Human Transcriptional Protein DPF3B Bound to A Histone H4 Peptide Containing N-Terminal Acetylation at Serine 1
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of Solution Structure of the Double Phd (Plant Homeodomain) Fingers of Human Transcriptional Protein DPF3B Bound to A Histone H4 Peptide Containing N-Terminal Acetylation at Serine 1 within 5.0Å range:
|
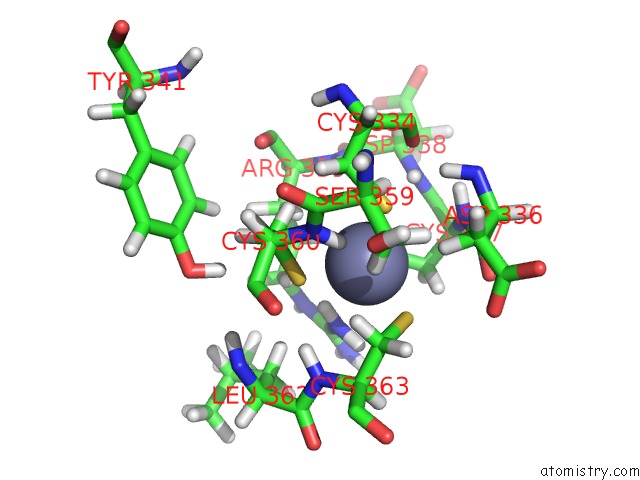
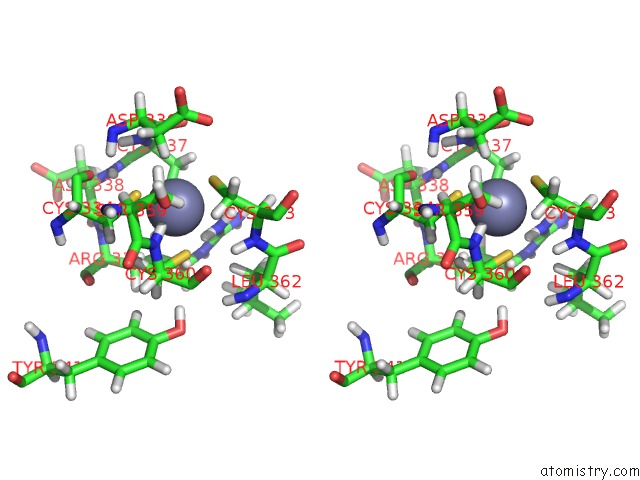
Zinc binding site 3 out of 4 in 2kwo
Go back to
Zinc binding site 3 out
of 4 in the Solution Structure of the Double Phd (Plant Homeodomain) Fingers of Human Transcriptional Protein DPF3B Bound to A Histone H4 Peptide Containing N-Terminal Acetylation at Serine 1
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 3 of Solution Structure of the Double Phd (Plant Homeodomain) Fingers of Human Transcriptional Protein DPF3B Bound to A Histone H4 Peptide Containing N-Terminal Acetylation at Serine 1 within 5.0Å range:
|
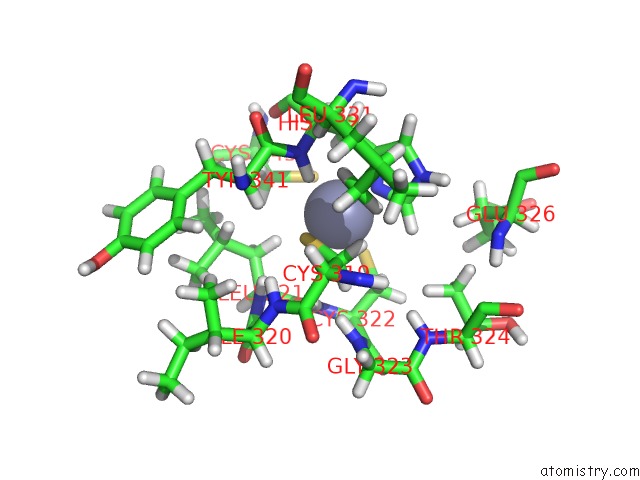
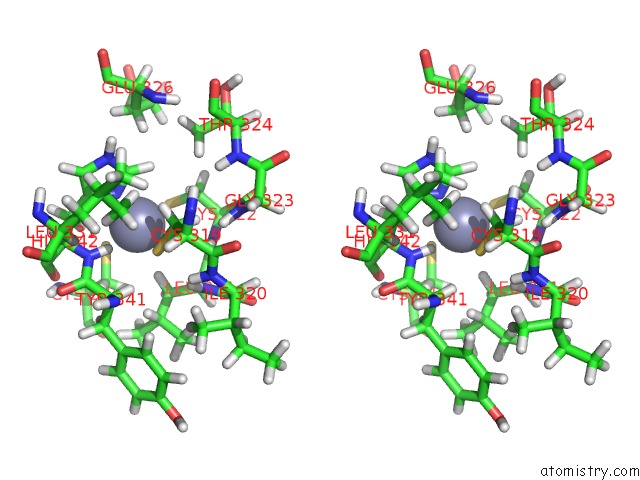
Zinc binding site 4 out of 4 in 2kwo
Go back to
Zinc binding site 4 out
of 4 in the Solution Structure of the Double Phd (Plant Homeodomain) Fingers of Human Transcriptional Protein DPF3B Bound to A Histone H4 Peptide Containing N-Terminal Acetylation at Serine 1
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 4 of Solution Structure of the Double Phd (Plant Homeodomain) Fingers of Human Transcriptional Protein DPF3B Bound to A Histone H4 Peptide Containing N-Terminal Acetylation at Serine 1 within 5.0Å range:
|
Reference:
L.Zeng,
Q.Zhang,
S.Li,
A.N.Plotnikov,
M.J.Walsh,
M.M.Zhou.
Mechanism and Regulation of Acetylated Histone Binding By the Tandem Phd Finger of DPF3B. Nature V. 466 258 2010.
ISSN: ISSN 0028-0836
PubMed: 20613843
DOI: 10.1038/NATURE09139
Page generated: Thu Oct 17 01:40:32 2024
ISSN: ISSN 0028-0836
PubMed: 20613843
DOI: 10.1038/NATURE09139
Last articles
Zn in 9J0NZn in 9J0O
Zn in 9J0P
Zn in 9FJX
Zn in 9EKB
Zn in 9C0F
Zn in 9CAH
Zn in 9CH0
Zn in 9CH3
Zn in 9CH1