Zinc »
PDB 2jun-2kem »
2k2g »
Zinc in PDB 2k2g: Solution Structure of the Wild-Type Catalytic Domain of Human Matrix Metalloproteinase 12 (Mmp-12) in Complex with A Tight-Binding Inhibitor
Enzymatic activity of Solution Structure of the Wild-Type Catalytic Domain of Human Matrix Metalloproteinase 12 (Mmp-12) in Complex with A Tight-Binding Inhibitor
All present enzymatic activity of Solution Structure of the Wild-Type Catalytic Domain of Human Matrix Metalloproteinase 12 (Mmp-12) in Complex with A Tight-Binding Inhibitor:
3.4.24.65;
3.4.24.65;
Zinc Binding Sites:
The binding sites of Zinc atom in the Solution Structure of the Wild-Type Catalytic Domain of Human Matrix Metalloproteinase 12 (Mmp-12) in Complex with A Tight-Binding Inhibitor
(pdb code 2k2g). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 2 binding sites of Zinc where determined in the Solution Structure of the Wild-Type Catalytic Domain of Human Matrix Metalloproteinase 12 (Mmp-12) in Complex with A Tight-Binding Inhibitor, PDB code: 2k2g:
Jump to Zinc binding site number: 1; 2;
In total 2 binding sites of Zinc where determined in the Solution Structure of the Wild-Type Catalytic Domain of Human Matrix Metalloproteinase 12 (Mmp-12) in Complex with A Tight-Binding Inhibitor, PDB code: 2k2g:
Jump to Zinc binding site number: 1; 2;
Zinc binding site 1 out of 2 in 2k2g
Go back to
Zinc binding site 1 out
of 2 in the Solution Structure of the Wild-Type Catalytic Domain of Human Matrix Metalloproteinase 12 (Mmp-12) in Complex with A Tight-Binding Inhibitor
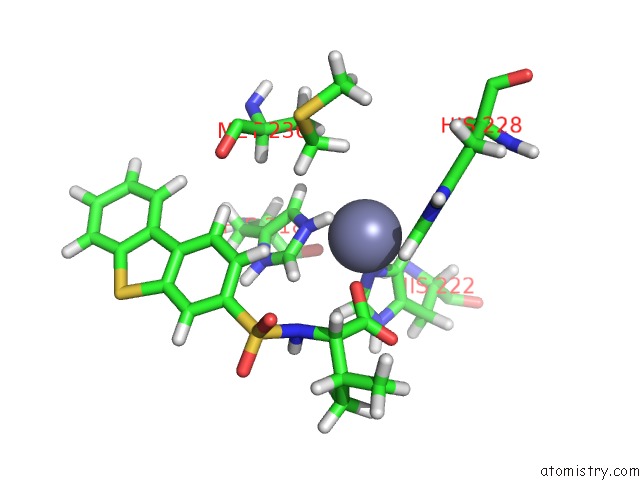
Mono view
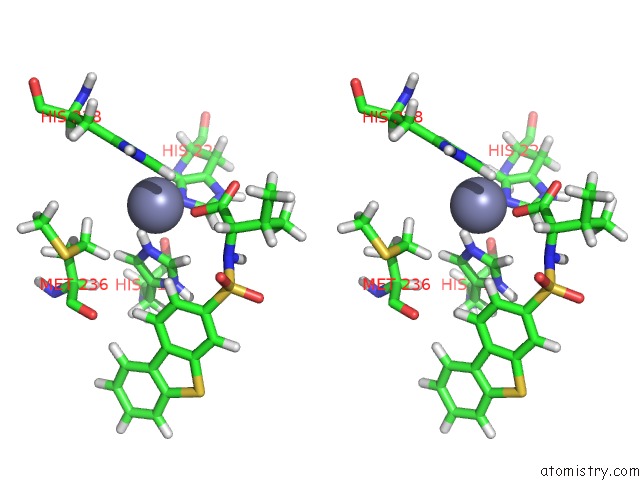
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Solution Structure of the Wild-Type Catalytic Domain of Human Matrix Metalloproteinase 12 (Mmp-12) in Complex with A Tight-Binding Inhibitor within 5.0Å range:
|
Zinc binding site 2 out of 2 in 2k2g
Go back to
Zinc binding site 2 out
of 2 in the Solution Structure of the Wild-Type Catalytic Domain of Human Matrix Metalloproteinase 12 (Mmp-12) in Complex with A Tight-Binding Inhibitor
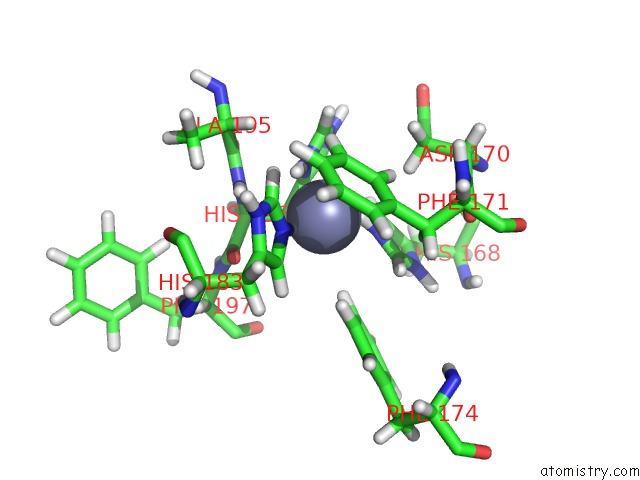
Mono view
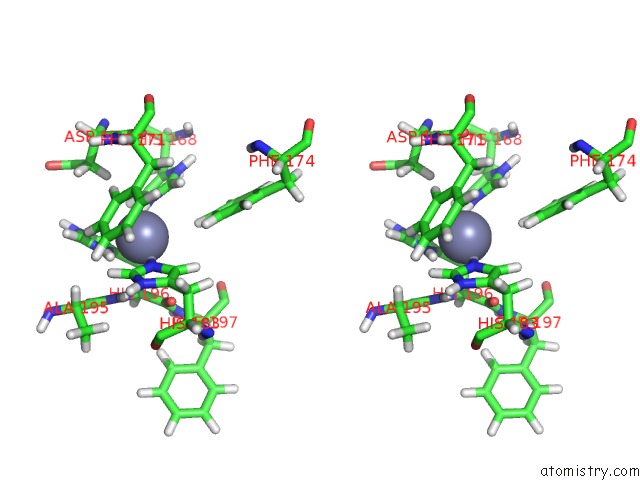
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of Solution Structure of the Wild-Type Catalytic Domain of Human Matrix Metalloproteinase 12 (Mmp-12) in Complex with A Tight-Binding Inhibitor within 5.0Å range:
|
Reference:
M.A.Markus,
B.Dwyer,
S.Wolfrom,
J.Li,
W.Li,
K.Malakian,
J.Wilhelm,
D.H.Tsao.
Solution Structure of Wild-Type Human Matrix Metalloproteinase 12 (Mmp-12) in Complex with A Tight-Binding Inhibitor. J.Biomol.uc(Nmr) V. 41 55 2008.
ISSN: ISSN 0925-2738
PubMed: 18425585
DOI: 10.1007/S10858-008-9236-4
Page generated: Thu Oct 17 01:30:30 2024
ISSN: ISSN 0925-2738
PubMed: 18425585
DOI: 10.1007/S10858-008-9236-4
Last articles
Zn in 9J0NZn in 9J0O
Zn in 9J0P
Zn in 9FJX
Zn in 9EKB
Zn in 9C0F
Zn in 9CAH
Zn in 9CH0
Zn in 9CH3
Zn in 9CH1