Zinc »
PDB 2gzi-2han »
2h3e »
Zinc in PDB 2h3e: Structure of Wild-Type E. Coli Aspartate Transcarbamoylase in the Presence of N-Phosphonacetyl-L-Isoasparagine at 2.3A Resolution
Enzymatic activity of Structure of Wild-Type E. Coli Aspartate Transcarbamoylase in the Presence of N-Phosphonacetyl-L-Isoasparagine at 2.3A Resolution
All present enzymatic activity of Structure of Wild-Type E. Coli Aspartate Transcarbamoylase in the Presence of N-Phosphonacetyl-L-Isoasparagine at 2.3A Resolution:
2.1.3.2;
2.1.3.2;
Protein crystallography data
The structure of Structure of Wild-Type E. Coli Aspartate Transcarbamoylase in the Presence of N-Phosphonacetyl-L-Isoasparagine at 2.3A Resolution, PDB code: 2h3e
was solved by
J.Eldo,
J.P.Cardia,
E.M.O'day,
J.Xia,
H.Tsuruta,
E.R.Kantrowitz,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 28.41 / 2.30 |
| Space group | P 3 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 120.320, 120.320, 154.510, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 20.6 / 25 |
Zinc Binding Sites:
The binding sites of Zinc atom in the Structure of Wild-Type E. Coli Aspartate Transcarbamoylase in the Presence of N-Phosphonacetyl-L-Isoasparagine at 2.3A Resolution
(pdb code 2h3e). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 2 binding sites of Zinc where determined in the Structure of Wild-Type E. Coli Aspartate Transcarbamoylase in the Presence of N-Phosphonacetyl-L-Isoasparagine at 2.3A Resolution, PDB code: 2h3e:
Jump to Zinc binding site number: 1; 2;
In total 2 binding sites of Zinc where determined in the Structure of Wild-Type E. Coli Aspartate Transcarbamoylase in the Presence of N-Phosphonacetyl-L-Isoasparagine at 2.3A Resolution, PDB code: 2h3e:
Jump to Zinc binding site number: 1; 2;
Zinc binding site 1 out of 2 in 2h3e
Go back to
Zinc binding site 1 out
of 2 in the Structure of Wild-Type E. Coli Aspartate Transcarbamoylase in the Presence of N-Phosphonacetyl-L-Isoasparagine at 2.3A Resolution
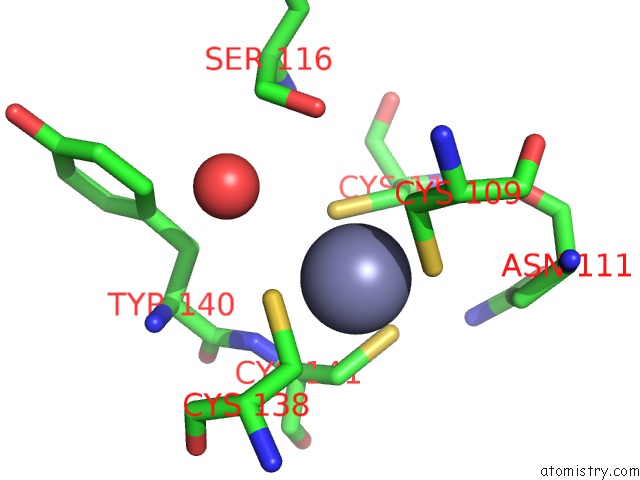
Mono view
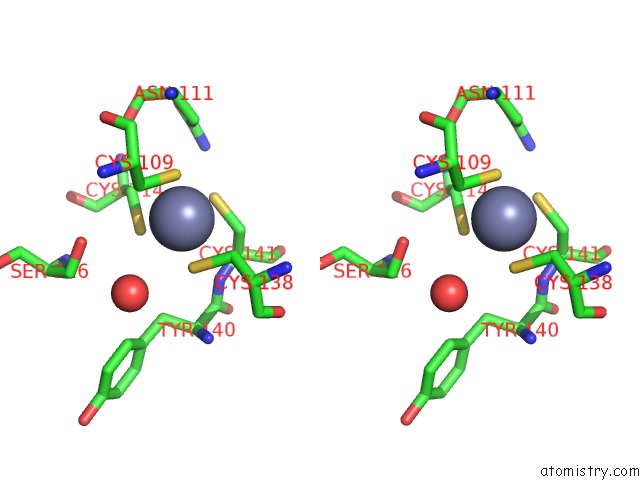
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Structure of Wild-Type E. Coli Aspartate Transcarbamoylase in the Presence of N-Phosphonacetyl-L-Isoasparagine at 2.3A Resolution within 5.0Å range:
|
Zinc binding site 2 out of 2 in 2h3e
Go back to
Zinc binding site 2 out
of 2 in the Structure of Wild-Type E. Coli Aspartate Transcarbamoylase in the Presence of N-Phosphonacetyl-L-Isoasparagine at 2.3A Resolution
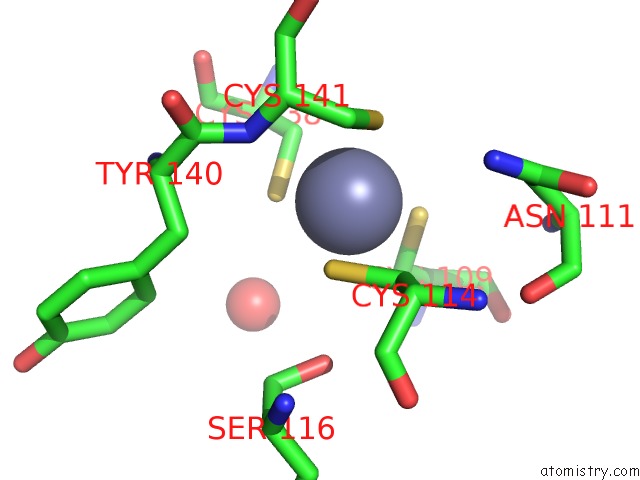
Mono view
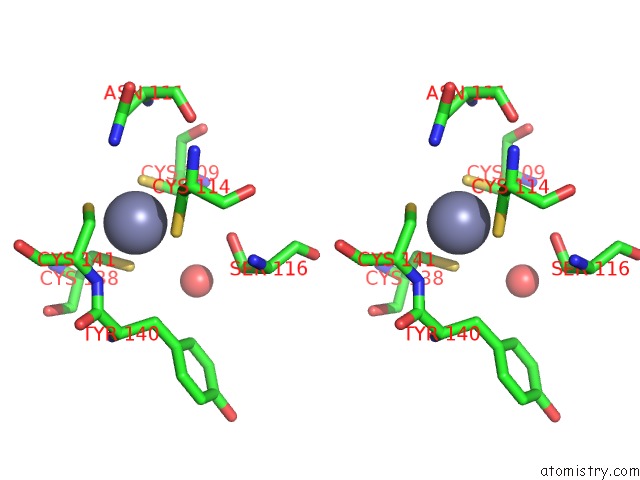
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of Structure of Wild-Type E. Coli Aspartate Transcarbamoylase in the Presence of N-Phosphonacetyl-L-Isoasparagine at 2.3A Resolution within 5.0Å range:
|
Reference:
J.Eldo,
J.P.Cardia,
E.M.O'day,
J.Xia,
H.Tsuruta,
E.R.Kantrowitz.
N-Phosphonacetyl-L-Isoasparagine A Potent and Specific Inhibitor of Escherichia Coli Aspartate Transcarbamoylase. J.Med.Chem. V. 49 5932 2006.
ISSN: ISSN 0022-2623
PubMed: 17004708
DOI: 10.1021/JM0607294
Page generated: Wed Aug 20 03:15:47 2025
ISSN: ISSN 0022-2623
PubMed: 17004708
DOI: 10.1021/JM0607294
Last articles
Zn in 3ADRZn in 3A6L
Zn in 3ADA
Zn in 3AD9
Zn in 3ABM
Zn in 3AD7
Zn in 3AD8
Zn in 3ABL
Zn in 3ABK
Zn in 3AAK