Zinc »
PDB 1y8q-1ykf »
1yb0 »
Zinc in PDB 1yb0: Structure of Plyl
Protein crystallography data
The structure of Structure of Plyl, PDB code: 1yb0
was solved by
L.Y.Low,
C.Yang,
M.Perego,
A.Osterman,
R.C.Liddington,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 29.24 / 1.86 |
| Space group | P 61 |
| Cell size a, b, c (Å), α, β, γ (°) | 163.181, 163.181, 37.292, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 20.6 / 24.2 |
Zinc Binding Sites:
The binding sites of Zinc atom in the Structure of Plyl
(pdb code 1yb0). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 3 binding sites of Zinc where determined in the Structure of Plyl, PDB code: 1yb0:
Jump to Zinc binding site number: 1; 2; 3;
In total 3 binding sites of Zinc where determined in the Structure of Plyl, PDB code: 1yb0:
Jump to Zinc binding site number: 1; 2; 3;
Zinc binding site 1 out of 3 in 1yb0
Go back to
Zinc binding site 1 out
of 3 in the Structure of Plyl

Mono view
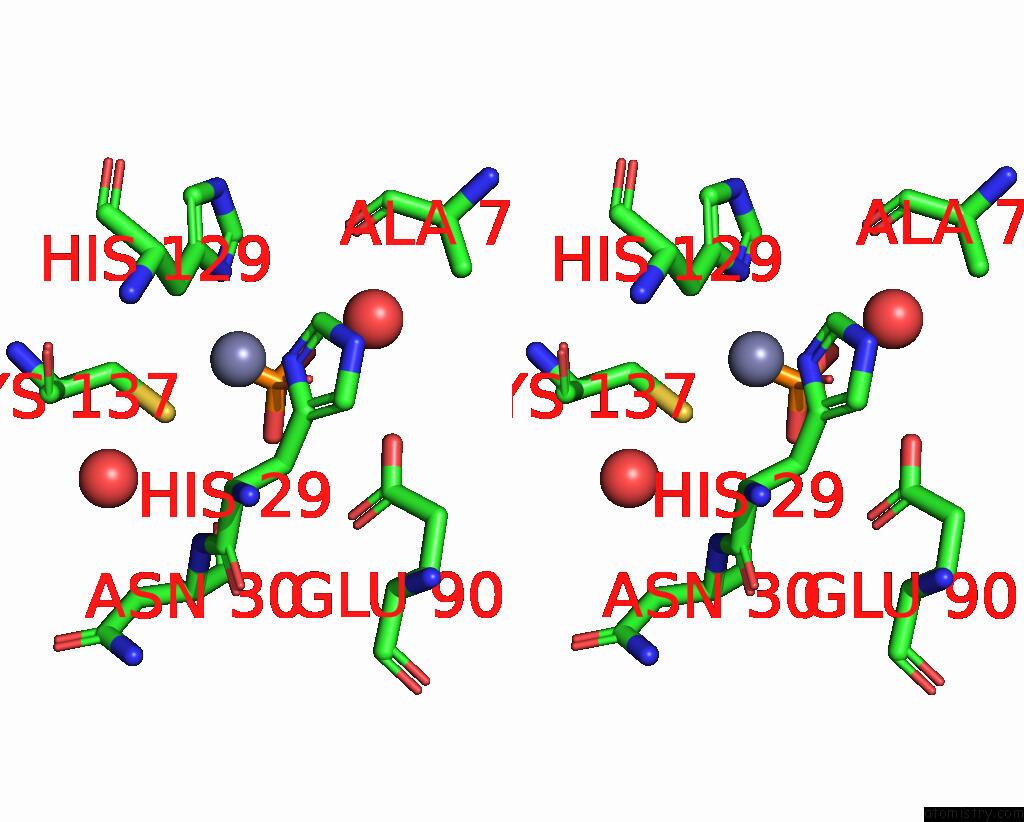
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Structure of Plyl within 5.0Å range:
|
Zinc binding site 2 out of 3 in 1yb0
Go back to
Zinc binding site 2 out
of 3 in the Structure of Plyl

Mono view
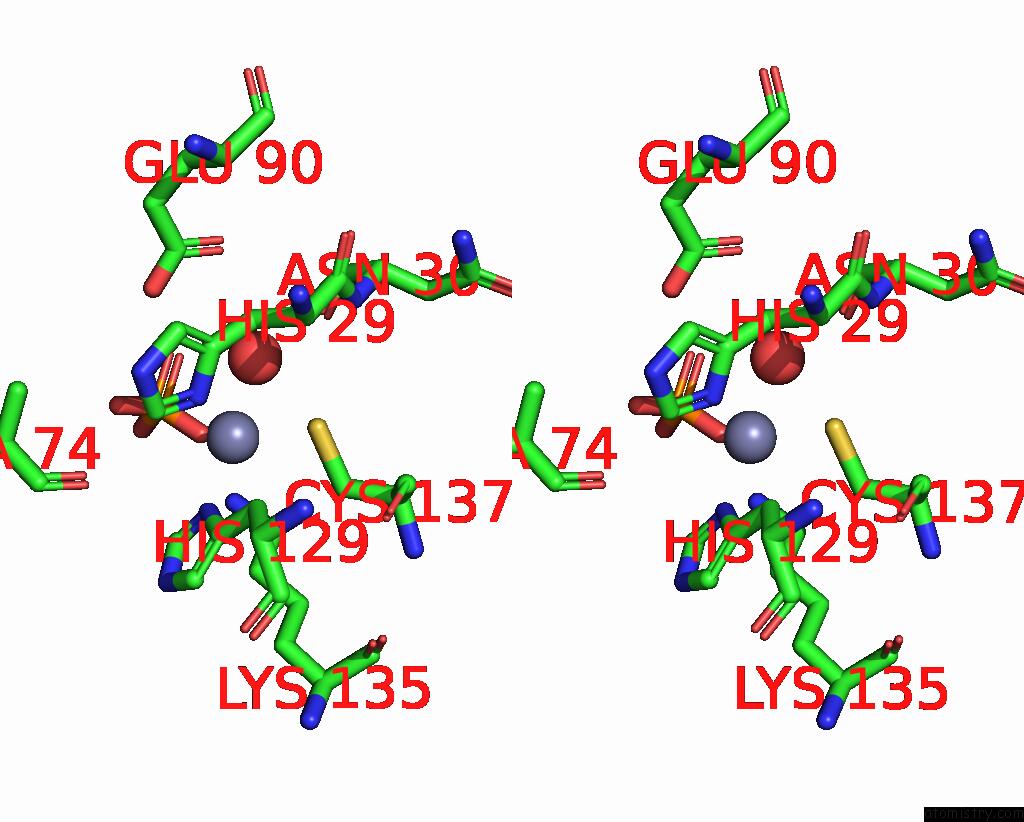
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of Structure of Plyl within 5.0Å range:
|
Zinc binding site 3 out of 3 in 1yb0
Go back to
Zinc binding site 3 out
of 3 in the Structure of Plyl

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 3 of Structure of Plyl within 5.0Å range:
|
Reference:
L.Y.Low,
C.Yang,
M.Perego,
A.Osterman,
R.C.Liddington.
Structure and Lytic Activity of A Bacillus Anthracis Prophage Endolysin J.Biol.Chem. V. 280 35433 2005.
ISSN: ISSN 0021-9258
PubMed: 16103125
DOI: 10.1074/JBC.M502723200
Page generated: Wed Aug 20 00:35:00 2025
ISSN: ISSN 0021-9258
PubMed: 16103125
DOI: 10.1074/JBC.M502723200
Last articles
Zn in 2NVUZn in 2NUT
Zn in 2NUP
Zn in 2NUV
Zn in 2NSO
Zn in 2NSF
Zn in 2NR6
Zn in 2NSE
Zn in 2NQJ
Zn in 2NNX