Zinc »
PDB 1x8h-1xm8 »
1xai »
Zinc in PDB 1xai: Crystal Structure of Staphlyococcus Aureus 3-Dehydroquinate Synthase (Dhqs) in Complex with ZN2+, Nad+ and Carbaphosphonate
Enzymatic activity of Crystal Structure of Staphlyococcus Aureus 3-Dehydroquinate Synthase (Dhqs) in Complex with ZN2+, Nad+ and Carbaphosphonate
All present enzymatic activity of Crystal Structure of Staphlyococcus Aureus 3-Dehydroquinate Synthase (Dhqs) in Complex with ZN2+, Nad+ and Carbaphosphonate:
4.2.3.4;
4.2.3.4;
Protein crystallography data
The structure of Crystal Structure of Staphlyococcus Aureus 3-Dehydroquinate Synthase (Dhqs) in Complex with ZN2+, Nad+ and Carbaphosphonate, PDB code: 1xai
was solved by
C.E.Nichols,
J.Ren,
K.Leslie,
B.Dhaliwal,
M.Lockyer,
I.Charles,
A.R.Hawkins,
D.K.Stammers,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 24.01 / 2.30 |
| Space group | P 43 |
| Cell size a, b, c (Å), α, β, γ (°) | 54.898, 54.898, 229.111, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 21.1 / 31.2 |
Zinc Binding Sites:
The binding sites of Zinc atom in the Crystal Structure of Staphlyococcus Aureus 3-Dehydroquinate Synthase (Dhqs) in Complex with ZN2+, Nad+ and Carbaphosphonate
(pdb code 1xai). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 2 binding sites of Zinc where determined in the Crystal Structure of Staphlyococcus Aureus 3-Dehydroquinate Synthase (Dhqs) in Complex with ZN2+, Nad+ and Carbaphosphonate, PDB code: 1xai:
Jump to Zinc binding site number: 1; 2;
In total 2 binding sites of Zinc where determined in the Crystal Structure of Staphlyococcus Aureus 3-Dehydroquinate Synthase (Dhqs) in Complex with ZN2+, Nad+ and Carbaphosphonate, PDB code: 1xai:
Jump to Zinc binding site number: 1; 2;
Zinc binding site 1 out of 2 in 1xai
Go back to
Zinc binding site 1 out
of 2 in the Crystal Structure of Staphlyococcus Aureus 3-Dehydroquinate Synthase (Dhqs) in Complex with ZN2+, Nad+ and Carbaphosphonate
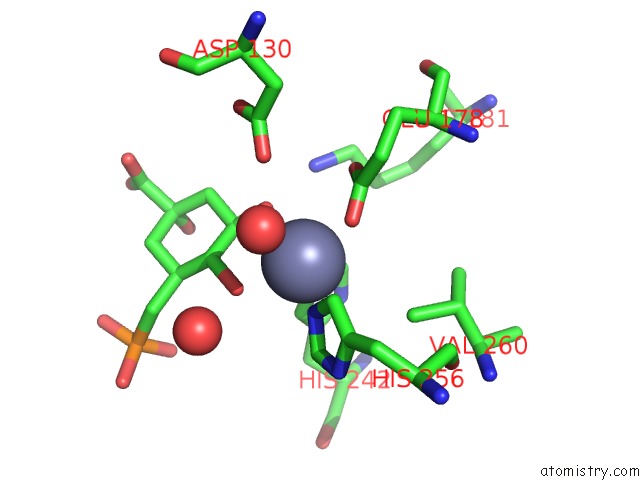
Mono view
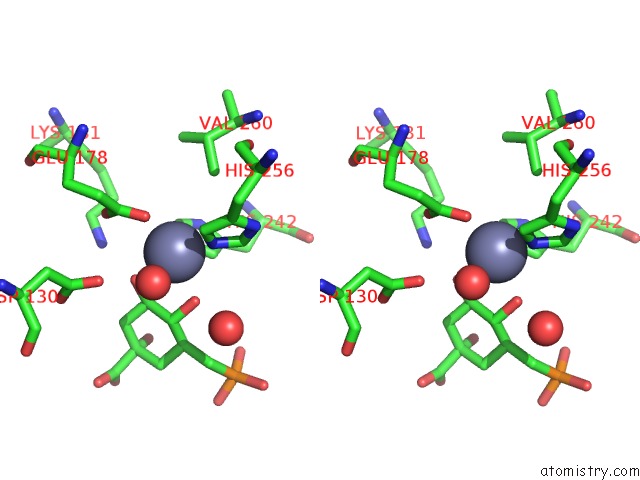
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Crystal Structure of Staphlyococcus Aureus 3-Dehydroquinate Synthase (Dhqs) in Complex with ZN2+, Nad+ and Carbaphosphonate within 5.0Å range:
|
Zinc binding site 2 out of 2 in 1xai
Go back to
Zinc binding site 2 out
of 2 in the Crystal Structure of Staphlyococcus Aureus 3-Dehydroquinate Synthase (Dhqs) in Complex with ZN2+, Nad+ and Carbaphosphonate
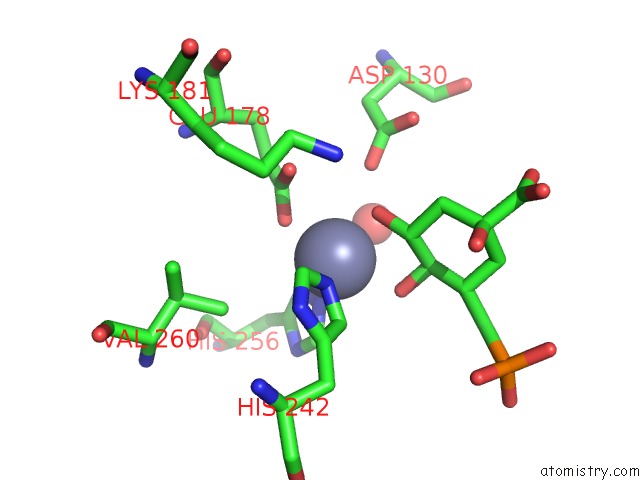
Mono view
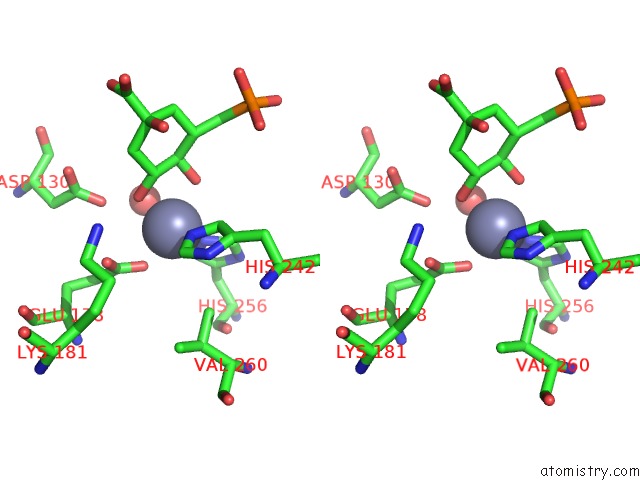
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of Crystal Structure of Staphlyococcus Aureus 3-Dehydroquinate Synthase (Dhqs) in Complex with ZN2+, Nad+ and Carbaphosphonate within 5.0Å range:
|
Reference:
C.E.Nichols,
J.Ren,
K.Leslie,
B.Dhaliwal,
M.Lockyer,
I.Charles,
A.R.Hawkins,
D.K.Stammers.
Comparison of Ligand Induced Conformational Changes and Domain Closure Mechanisms, Between Prokaryotic and Eukaryotic Dehydroquinate Synthases. J.Mol.Biol. V. 343 533 2004.
ISSN: ISSN 0022-2836
PubMed: 15465043
DOI: 10.1016/J.JMB.2004.08.039
Page generated: Wed Aug 20 00:14:09 2025
ISSN: ISSN 0022-2836
PubMed: 15465043
DOI: 10.1016/J.JMB.2004.08.039
Last articles
Zn in 2GFOZn in 2GEQ
Zn in 2GEH
Zn in 2GA6
Zn in 2G9T
Zn in 2GDA
Zn in 2GC3
Zn in 2GD8
Zn in 2GC2
Zn in 2GC1