Zinc »
PDB 1kzy-1lg6 »
1ld8 »
Zinc in PDB 1ld8: Co-Crystal Structure of Human Farnesyltransferase with Farnesyldiphosphate and Inhibitor Compound 49
Protein crystallography data
The structure of Co-Crystal Structure of Human Farnesyltransferase with Farnesyldiphosphate and Inhibitor Compound 49, PDB code: 1ld8
was solved by
J.S.Taylor,
K.L.Terry,
L.S.Beese,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 30.85 / 1.80 |
| Space group | P 61 |
| Cell size a, b, c (Å), α, β, γ (°) | 178.134, 178.134, 64.461, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 18.4 / 20.2 |
Zinc Binding Sites:
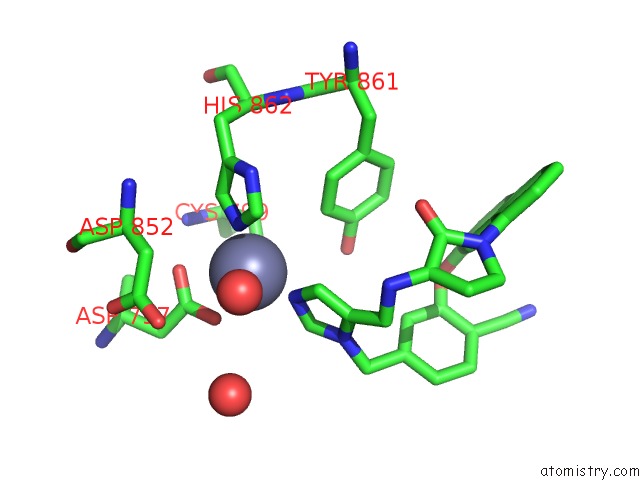
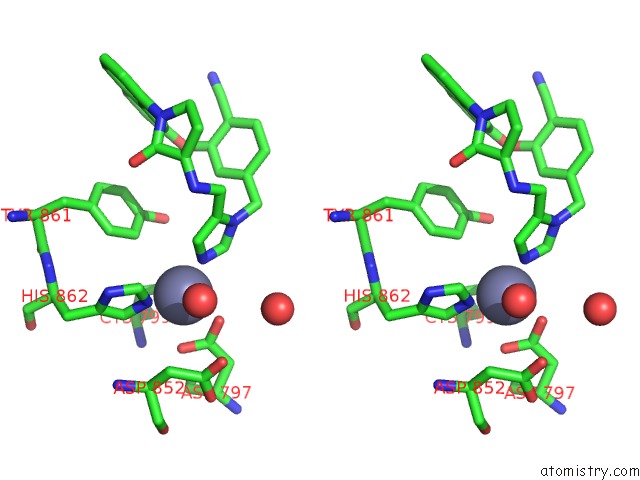
The binding sites of Zinc atom in the Co-Crystal Structure of Human Farnesyltransferase with Farnesyldiphosphate and Inhibitor Compound 49
(pdb code 1ld8). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total only one binding site of Zinc was determined in the Co-Crystal Structure of Human Farnesyltransferase with Farnesyldiphosphate and Inhibitor Compound 49, PDB code: 1ld8:
In total only one binding site of Zinc was determined in the Co-Crystal Structure of Human Farnesyltransferase with Farnesyldiphosphate and Inhibitor Compound 49, PDB code: 1ld8:
Zinc binding site 1 out of 1 in 1ld8
Go back to
Zinc binding site 1 out
of 1 in the Co-Crystal Structure of Human Farnesyltransferase with Farnesyldiphosphate and Inhibitor Compound 49
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Co-Crystal Structure of Human Farnesyltransferase with Farnesyldiphosphate and Inhibitor Compound 49 within 5.0Å range:
|
Reference:
I.M.Bell,
S.N.Gallicchio,
M.Abrams,
L.S.Beese,
D.C.Beshore,
H.Bhimnathwala,
M.J.Bogusky,
C.A.Buser,
J.C.Culberson,
J.Davide,
M.Ellis-Hutchings,
C.Fernandes,
J.B.Gibbs,
S.L.Graham,
K.A.Hamilton,
G.D.Hartman,
D.C.Heimbrook,
C.F.Homnick,
H.E.Huber,
J.R.Huff,
K.Kassahun,
K.S.Koblan,
N.E.Kohl,
R.B.Lobell,
J.J.Lynch Jr.,
R.Robinson,
A.D.Rodrigues,
J.S.Taylor,
E.S.Walsh,
T.M.Williams,
C.B.Zartman.
3-Aminopyrrolidinone Farnesyltransferase Inhibitors: Design of Macrocyclic Compounds with Improved Pharmacokinetics and Excellent Cell Potency. J.Med.Chem. V. 45 2388 2002.
ISSN: ISSN 0022-2623
PubMed: 12036349
DOI: 10.1021/JM010531D
Page generated: Tue Aug 19 21:31:18 2025
ISSN: ISSN 0022-2623
PubMed: 12036349
DOI: 10.1021/JM010531D
Last articles
Zn in 1WYHZn in 1WWR
Zn in 1WXO
Zn in 1WXZ
Zn in 1WXY
Zn in 1WWG
Zn in 1WWE
Zn in 1WWF
Zn in 1WW1
Zn in 1WWD