Zinc »
PDB 8xvs-8yha »
8yag »
Zinc in PDB 8yag: Cryo-Electron Microscopic Structure of An Amide Hydrolase From Pseudoxanthomonas Wuyuanensis
Zinc Binding Sites:
Pages:
>>> Page 1 <<< Page 2, Binding sites: 11 - 16;Binding sites:
The binding sites of Zinc atom in the Cryo-Electron Microscopic Structure of An Amide Hydrolase From Pseudoxanthomonas Wuyuanensis (pdb code 8yag). This binding sites where shown within 5.0 Angstroms radius around Zinc atom.In total 16 binding sites of Zinc where determined in the Cryo-Electron Microscopic Structure of An Amide Hydrolase From Pseudoxanthomonas Wuyuanensis, PDB code: 8yag:
Jump to Zinc binding site number: 1; 2; 3; 4; 5; 6; 7; 8; 9; 10;
Zinc binding site 1 out of 16 in 8yag
Go back to
Zinc binding site 1 out
of 16 in the Cryo-Electron Microscopic Structure of An Amide Hydrolase From Pseudoxanthomonas Wuyuanensis
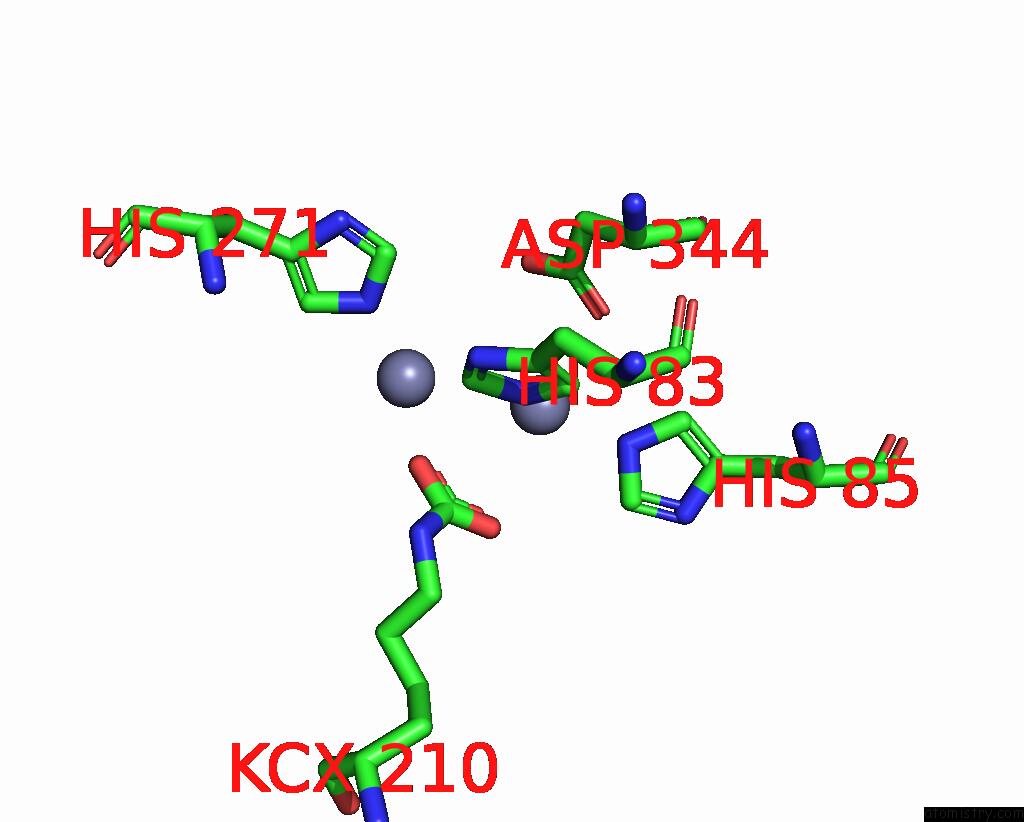
Mono view
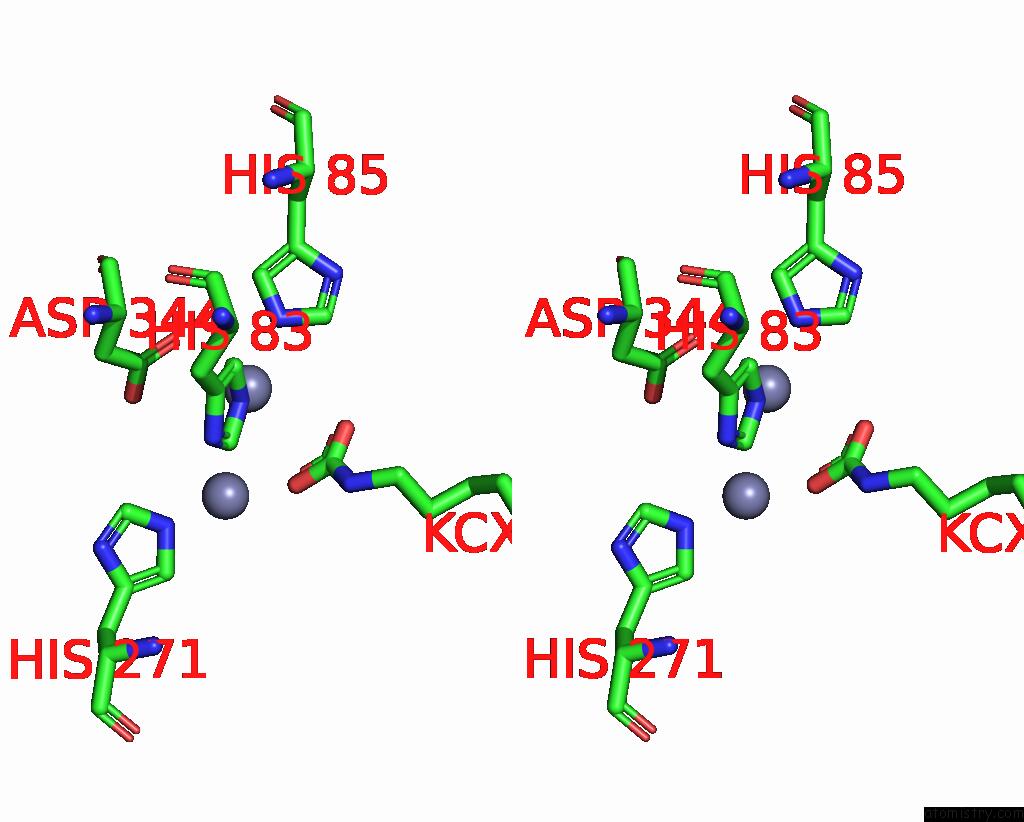
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Cryo-Electron Microscopic Structure of An Amide Hydrolase From Pseudoxanthomonas Wuyuanensis within 5.0Å range:
|
Zinc binding site 2 out of 16 in 8yag
Go back to
Zinc binding site 2 out
of 16 in the Cryo-Electron Microscopic Structure of An Amide Hydrolase From Pseudoxanthomonas Wuyuanensis
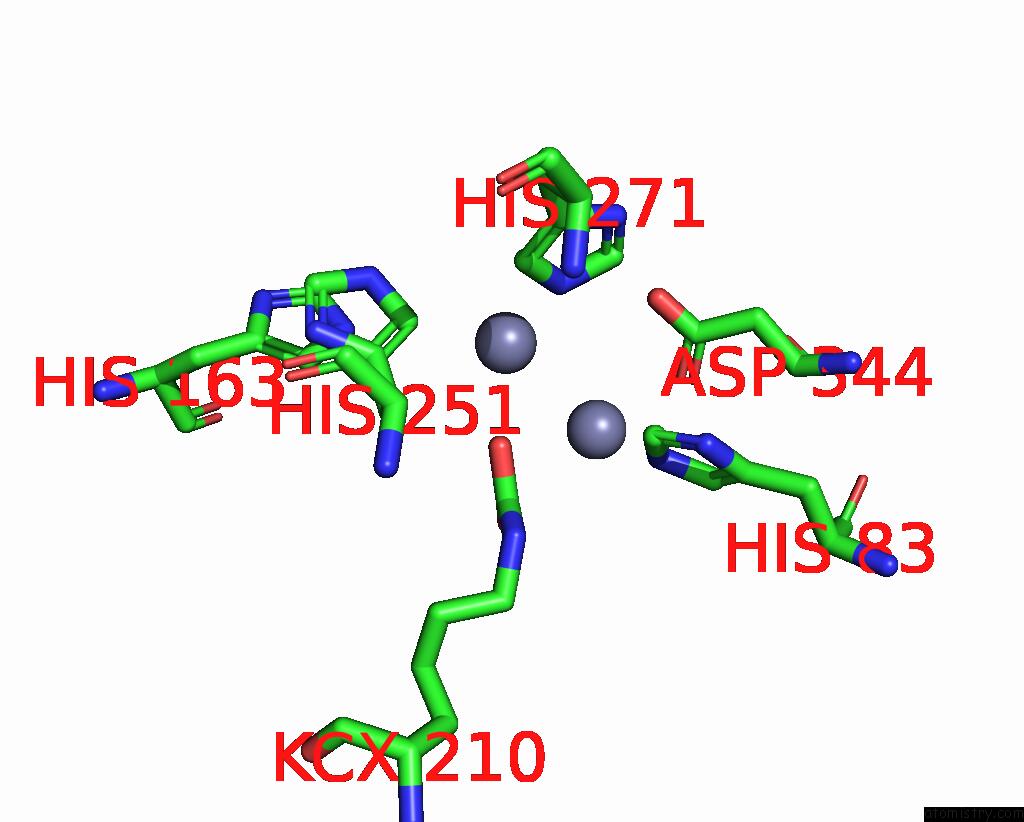
Mono view
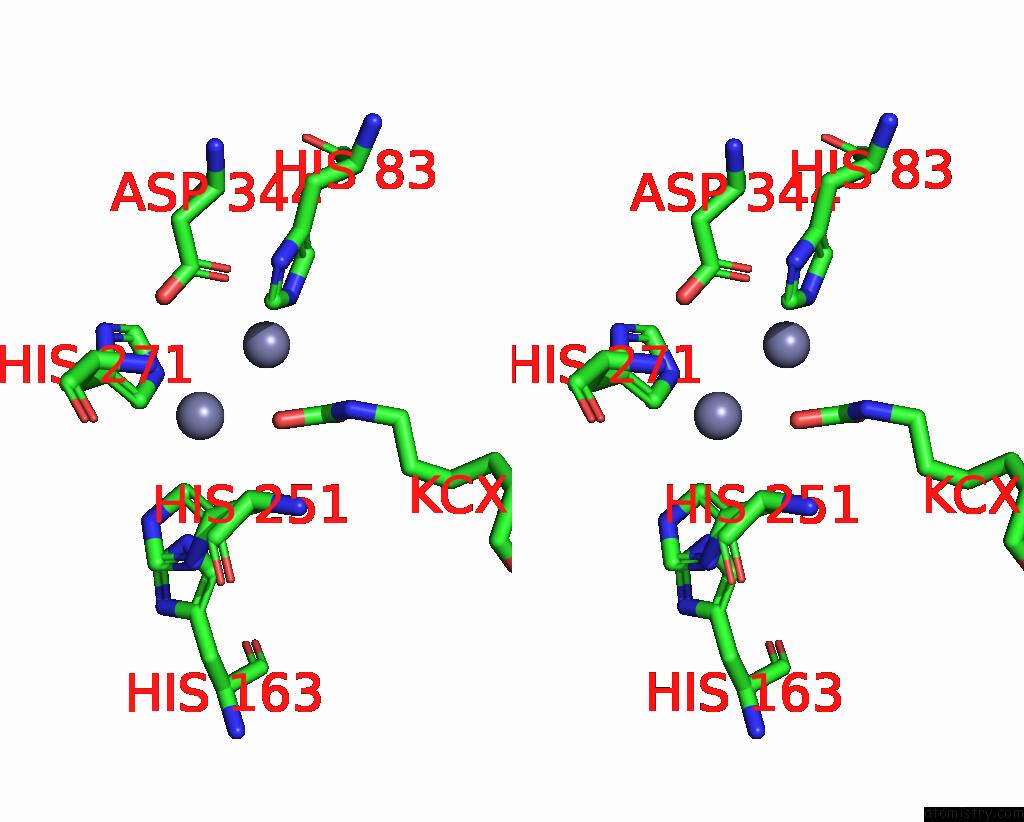
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of Cryo-Electron Microscopic Structure of An Amide Hydrolase From Pseudoxanthomonas Wuyuanensis within 5.0Å range:
|
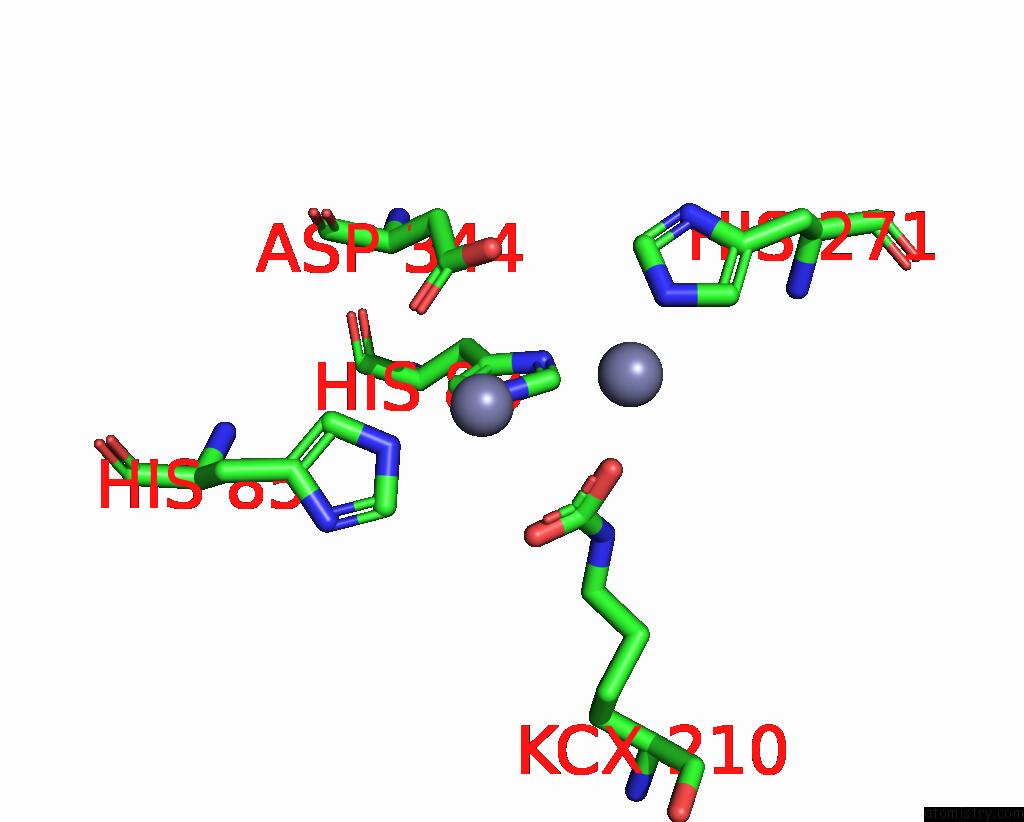
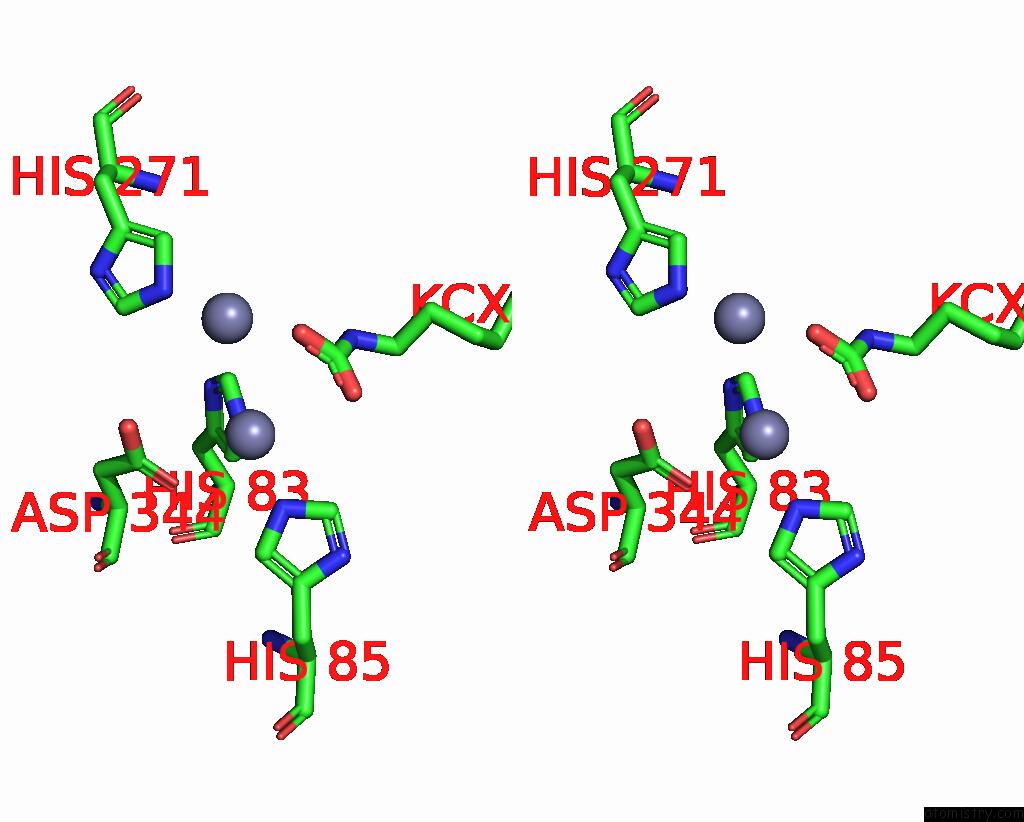
Zinc binding site 3 out of 16 in 8yag
Go back to
Zinc binding site 3 out
of 16 in the Cryo-Electron Microscopic Structure of An Amide Hydrolase From Pseudoxanthomonas Wuyuanensis
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 3 of Cryo-Electron Microscopic Structure of An Amide Hydrolase From Pseudoxanthomonas Wuyuanensis within 5.0Å range:
|
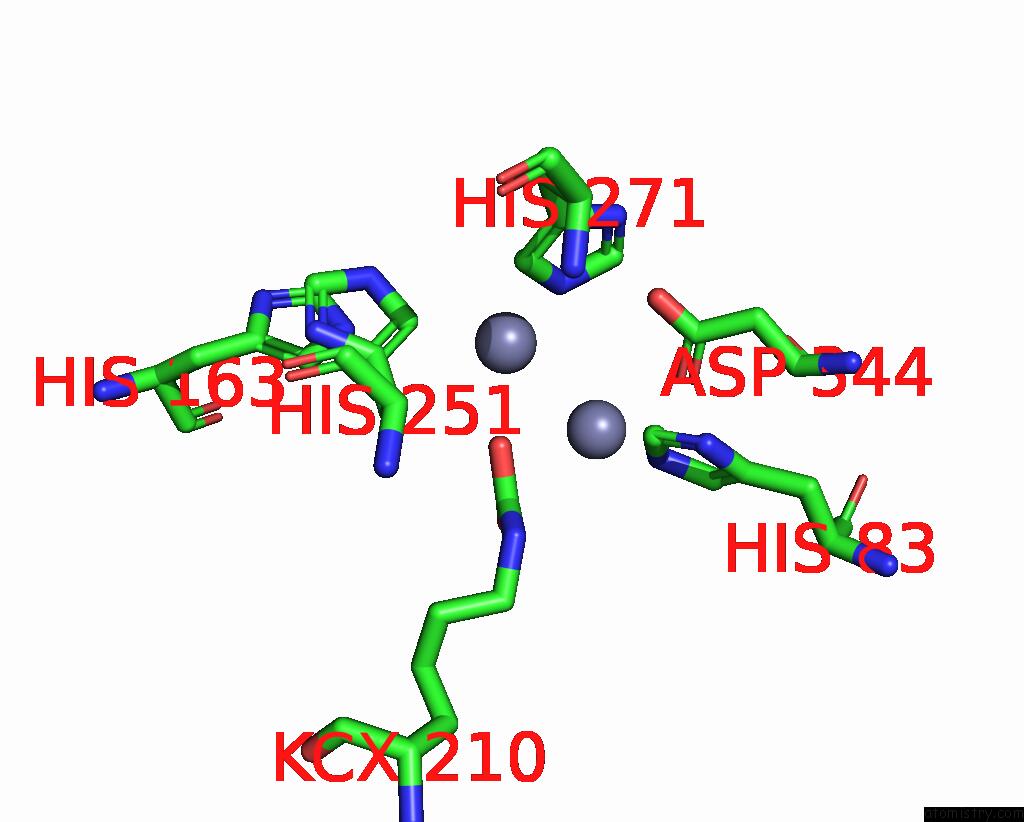
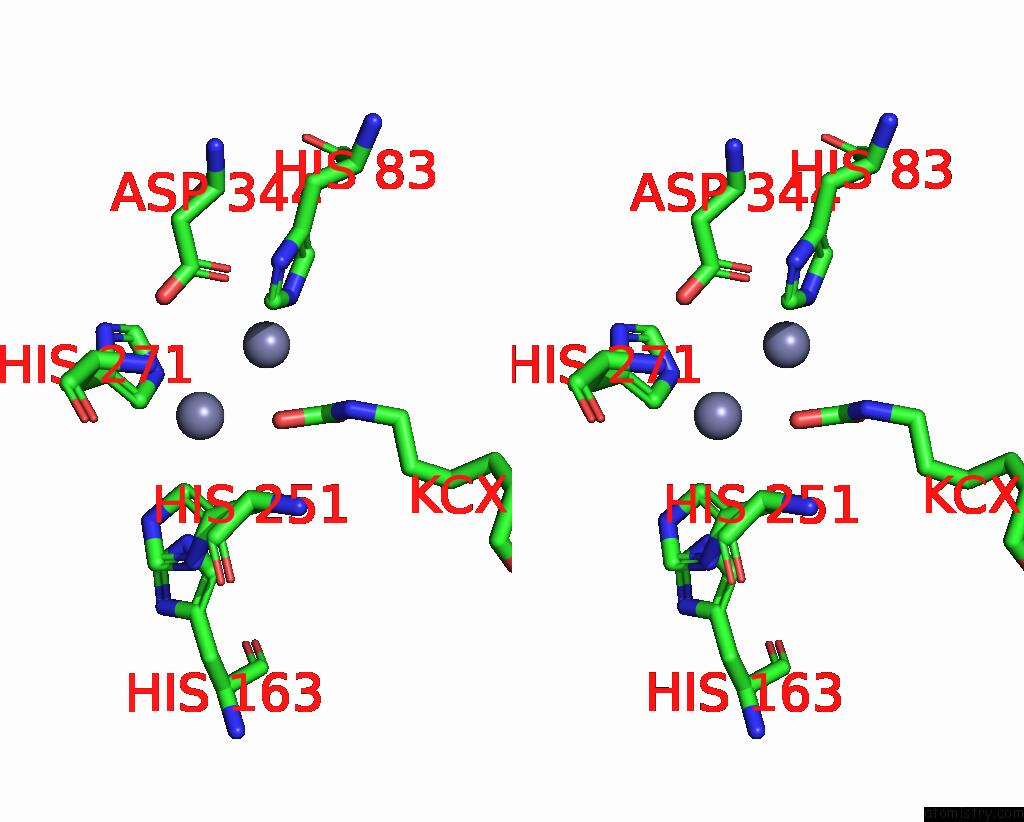
Zinc binding site 4 out of 16 in 8yag
Go back to
Zinc binding site 4 out
of 16 in the Cryo-Electron Microscopic Structure of An Amide Hydrolase From Pseudoxanthomonas Wuyuanensis
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 4 of Cryo-Electron Microscopic Structure of An Amide Hydrolase From Pseudoxanthomonas Wuyuanensis within 5.0Å range:
|
Zinc binding site 5 out of 16 in 8yag
Go back to
Zinc binding site 5 out
of 16 in the Cryo-Electron Microscopic Structure of An Amide Hydrolase From Pseudoxanthomonas Wuyuanensis
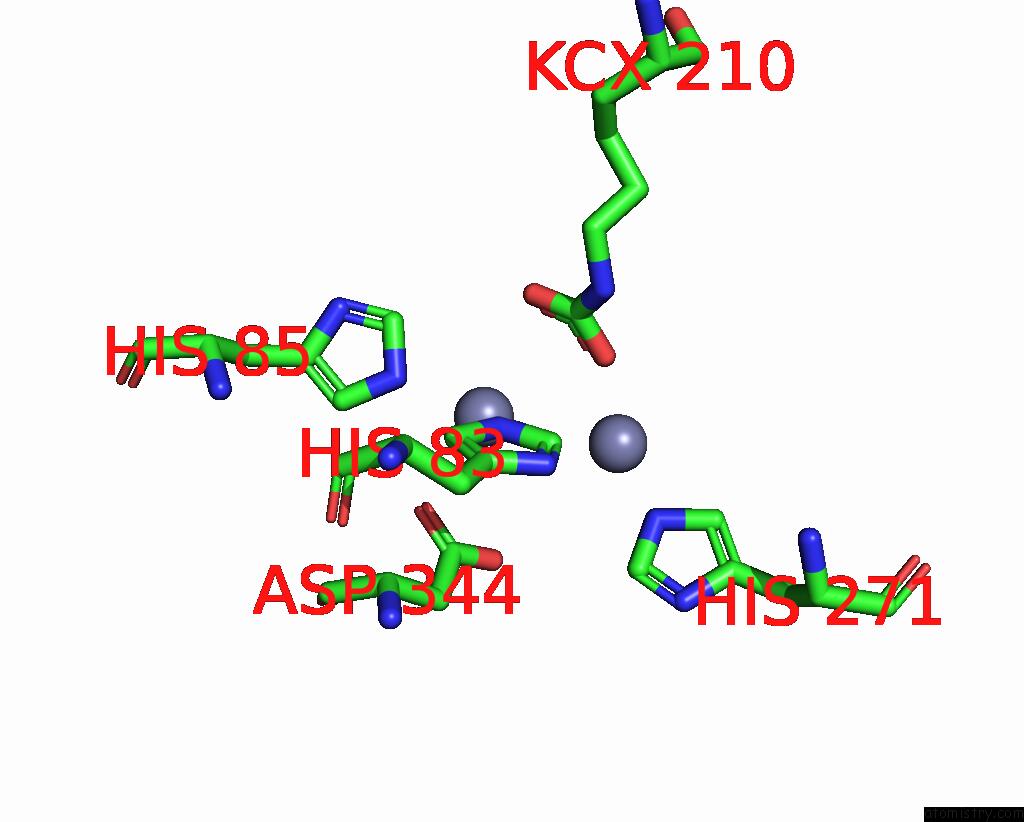
Mono view
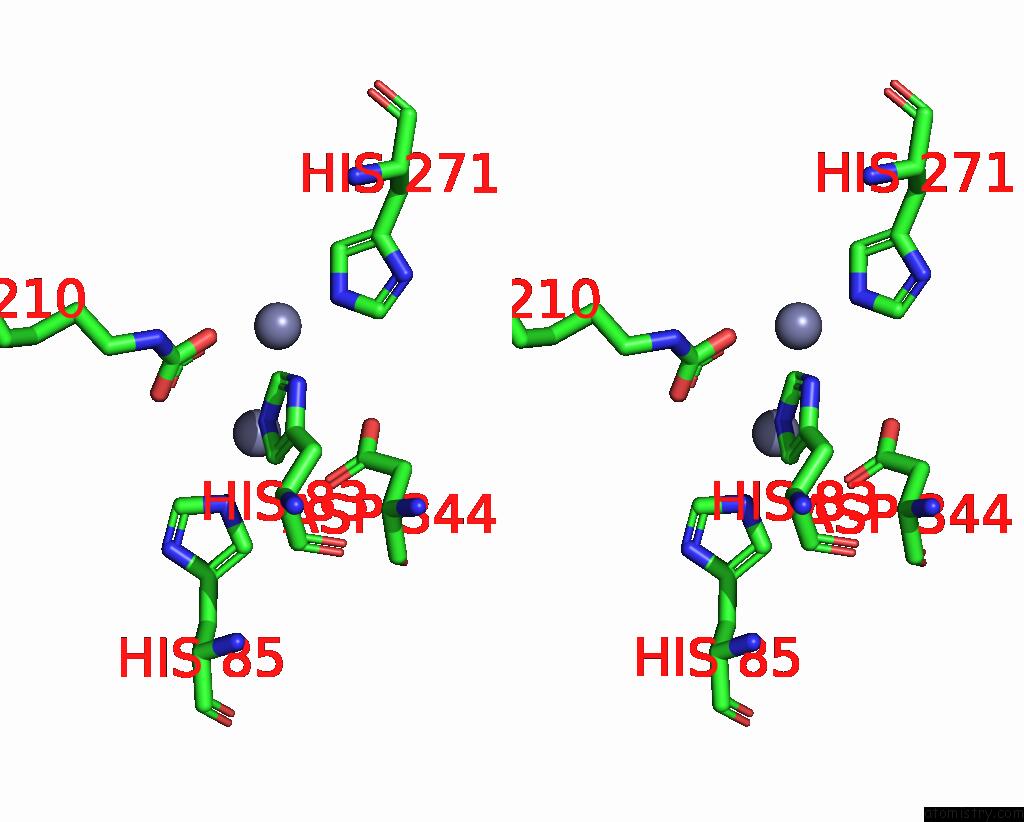
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 5 of Cryo-Electron Microscopic Structure of An Amide Hydrolase From Pseudoxanthomonas Wuyuanensis within 5.0Å range:
|
Zinc binding site 6 out of 16 in 8yag
Go back to
Zinc binding site 6 out
of 16 in the Cryo-Electron Microscopic Structure of An Amide Hydrolase From Pseudoxanthomonas Wuyuanensis
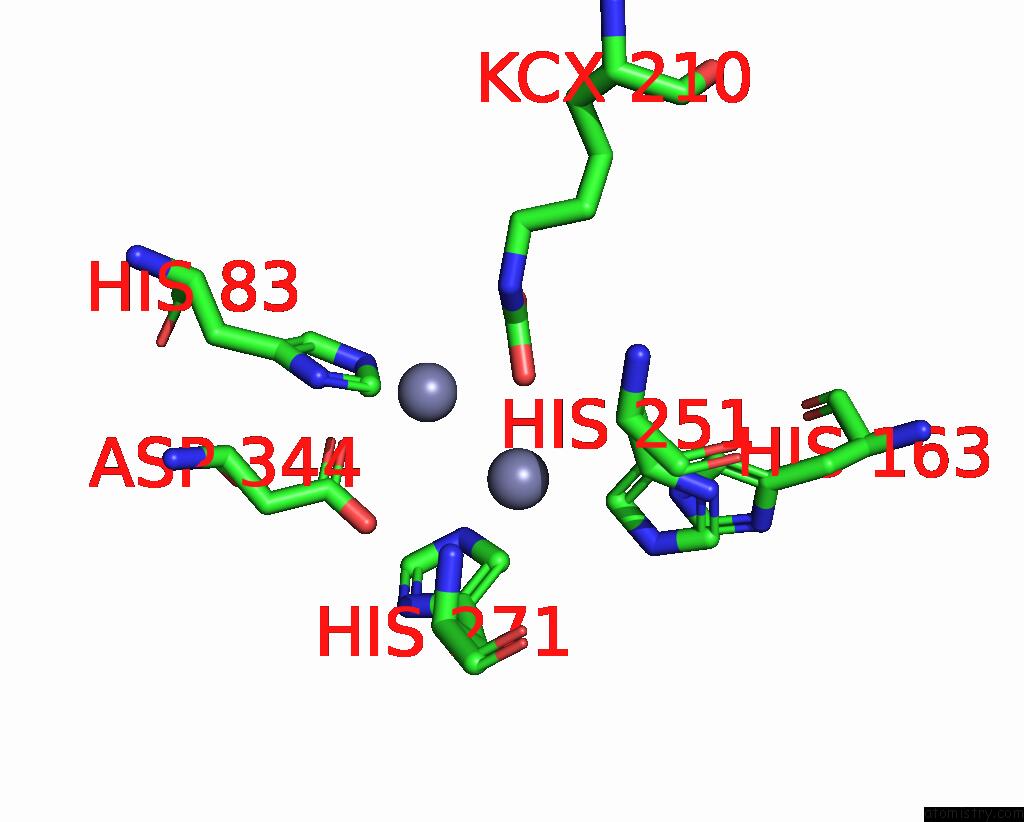
Mono view
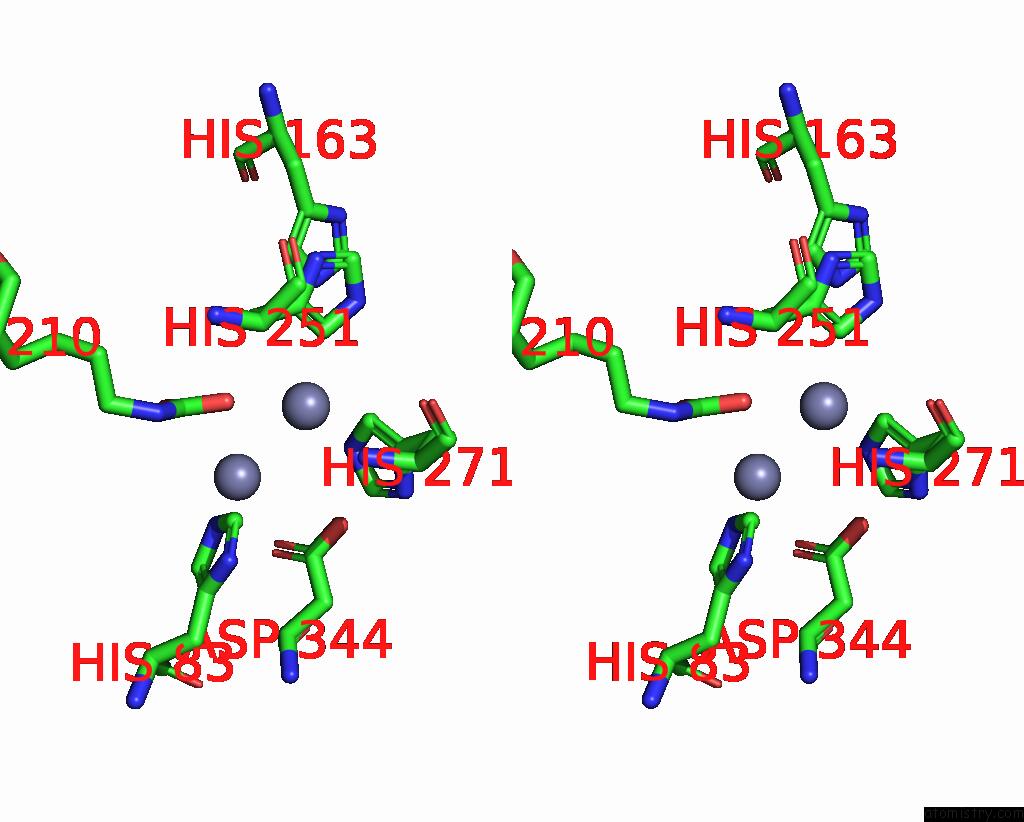
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 6 of Cryo-Electron Microscopic Structure of An Amide Hydrolase From Pseudoxanthomonas Wuyuanensis within 5.0Å range:
|
Zinc binding site 7 out of 16 in 8yag
Go back to
Zinc binding site 7 out
of 16 in the Cryo-Electron Microscopic Structure of An Amide Hydrolase From Pseudoxanthomonas Wuyuanensis
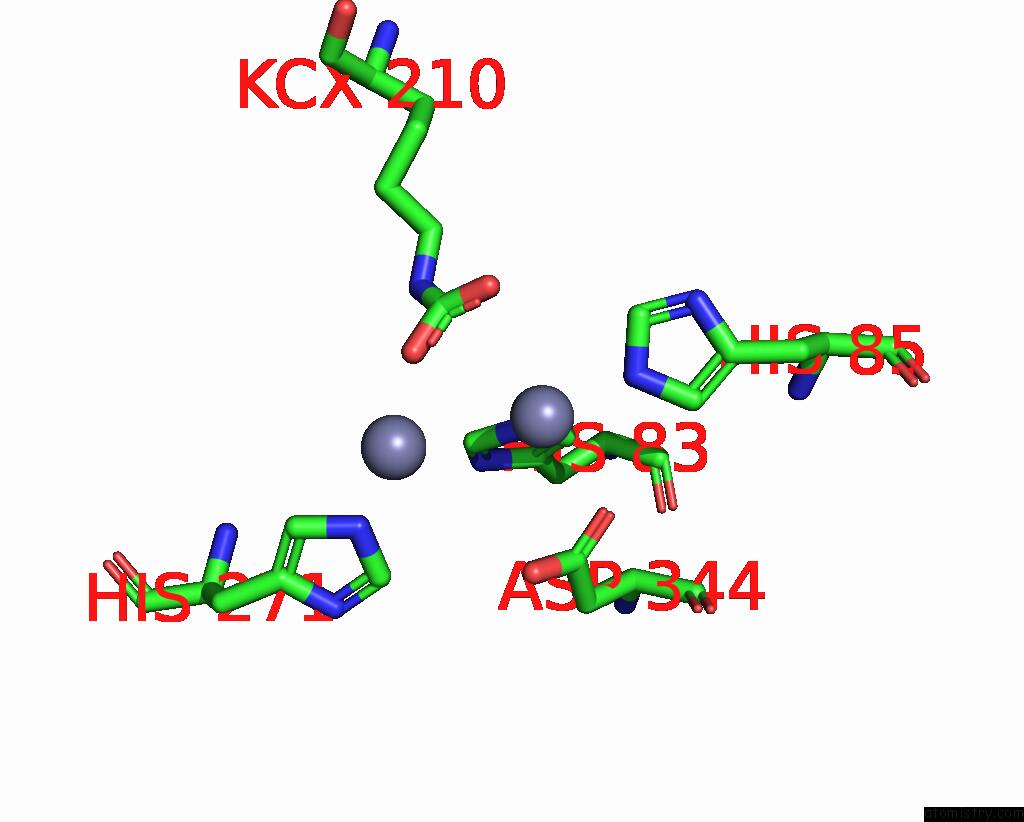
Mono view
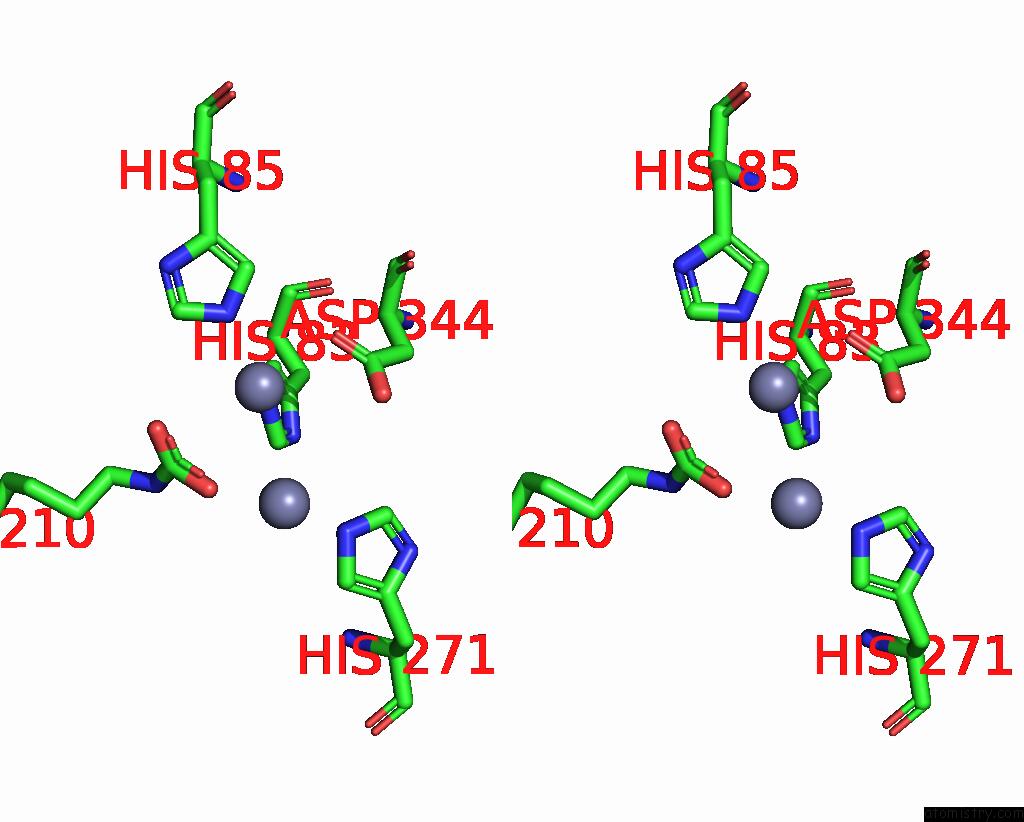
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 7 of Cryo-Electron Microscopic Structure of An Amide Hydrolase From Pseudoxanthomonas Wuyuanensis within 5.0Å range:
|
Zinc binding site 8 out of 16 in 8yag
Go back to
Zinc binding site 8 out
of 16 in the Cryo-Electron Microscopic Structure of An Amide Hydrolase From Pseudoxanthomonas Wuyuanensis
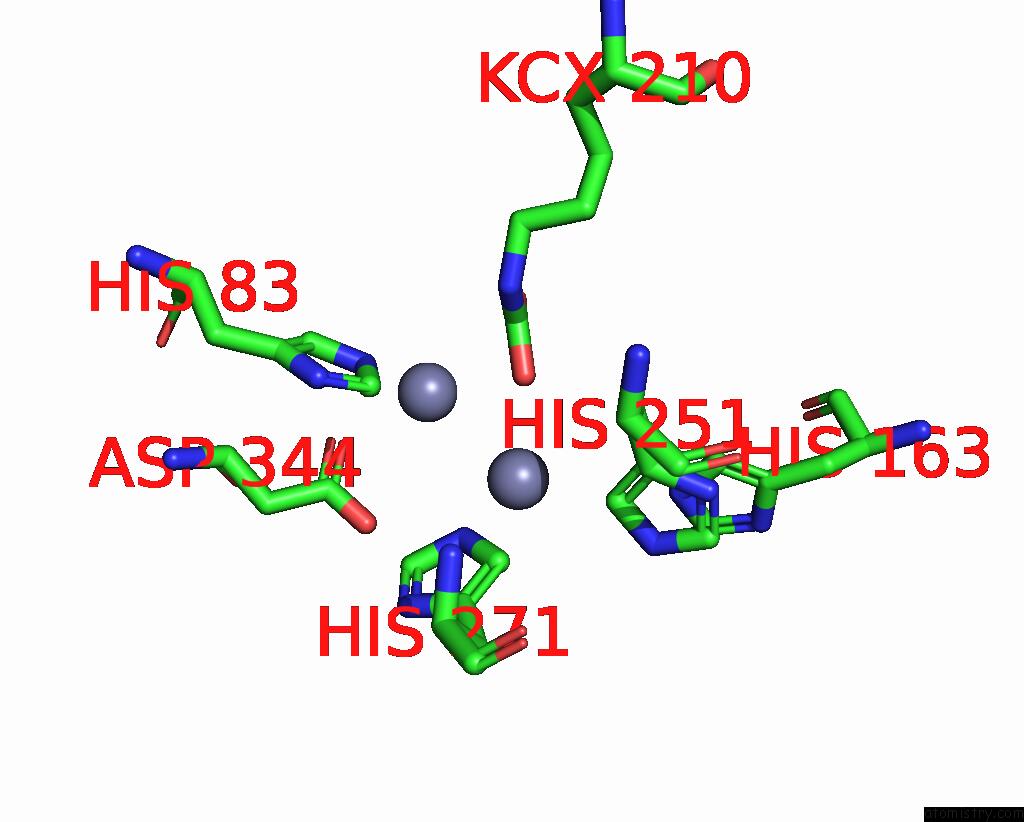
Mono view
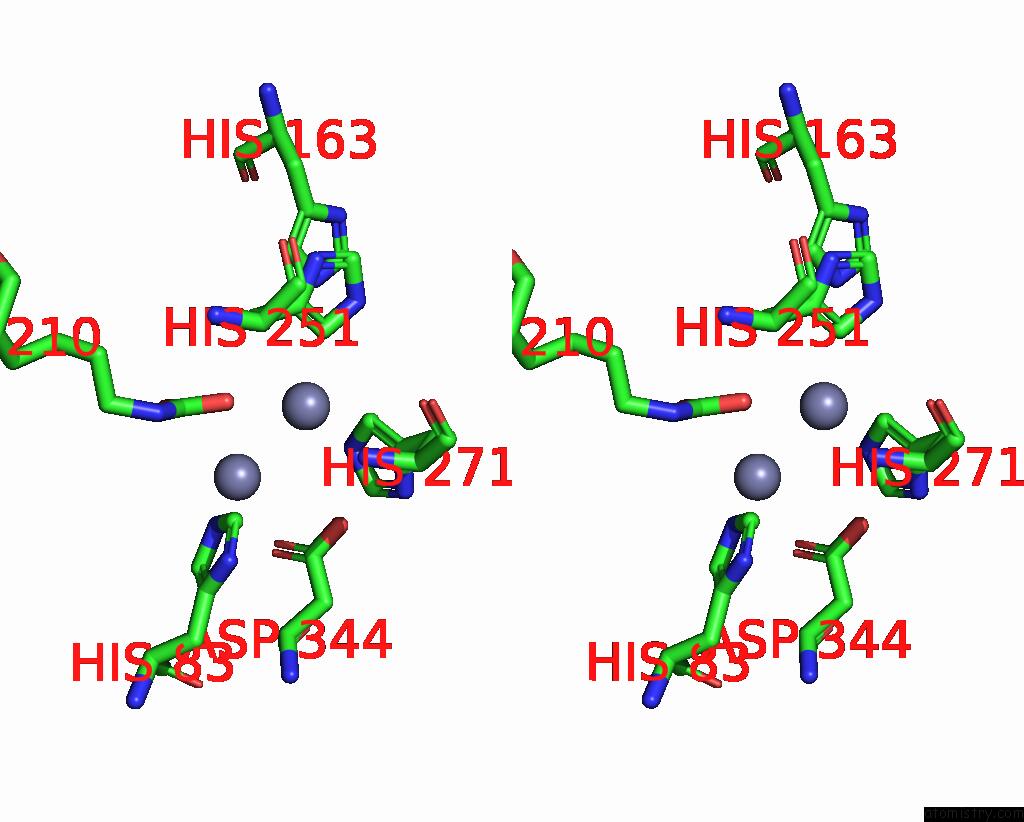
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 8 of Cryo-Electron Microscopic Structure of An Amide Hydrolase From Pseudoxanthomonas Wuyuanensis within 5.0Å range:
|
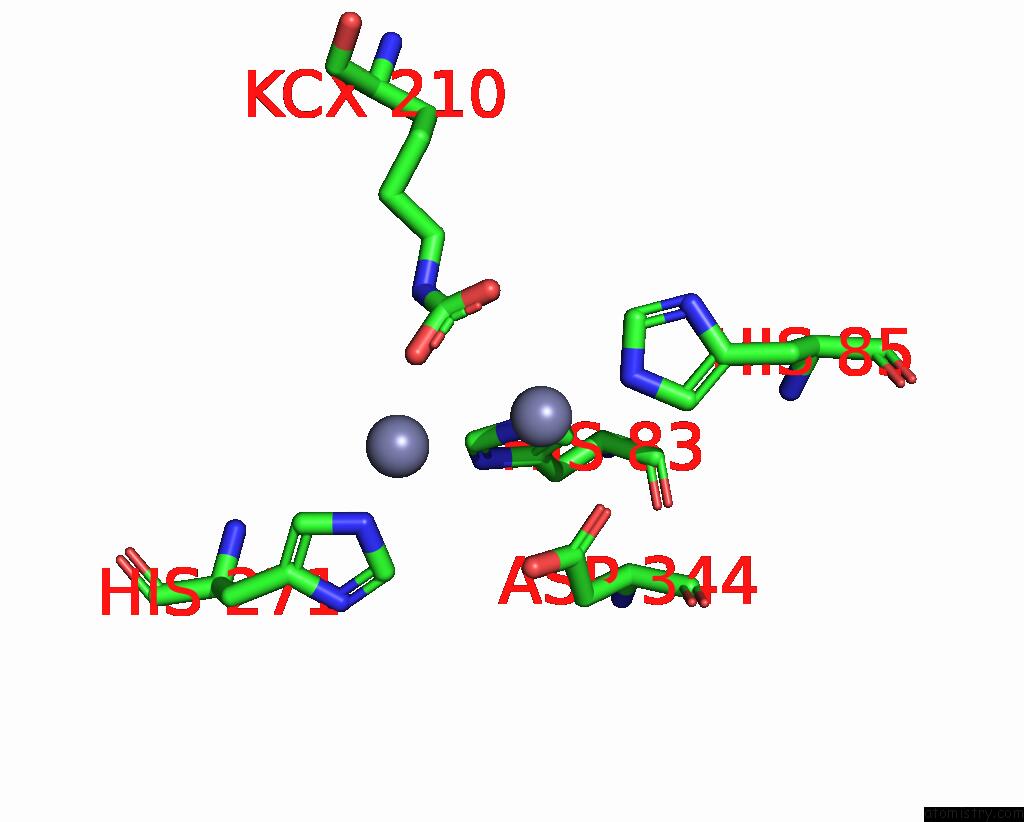
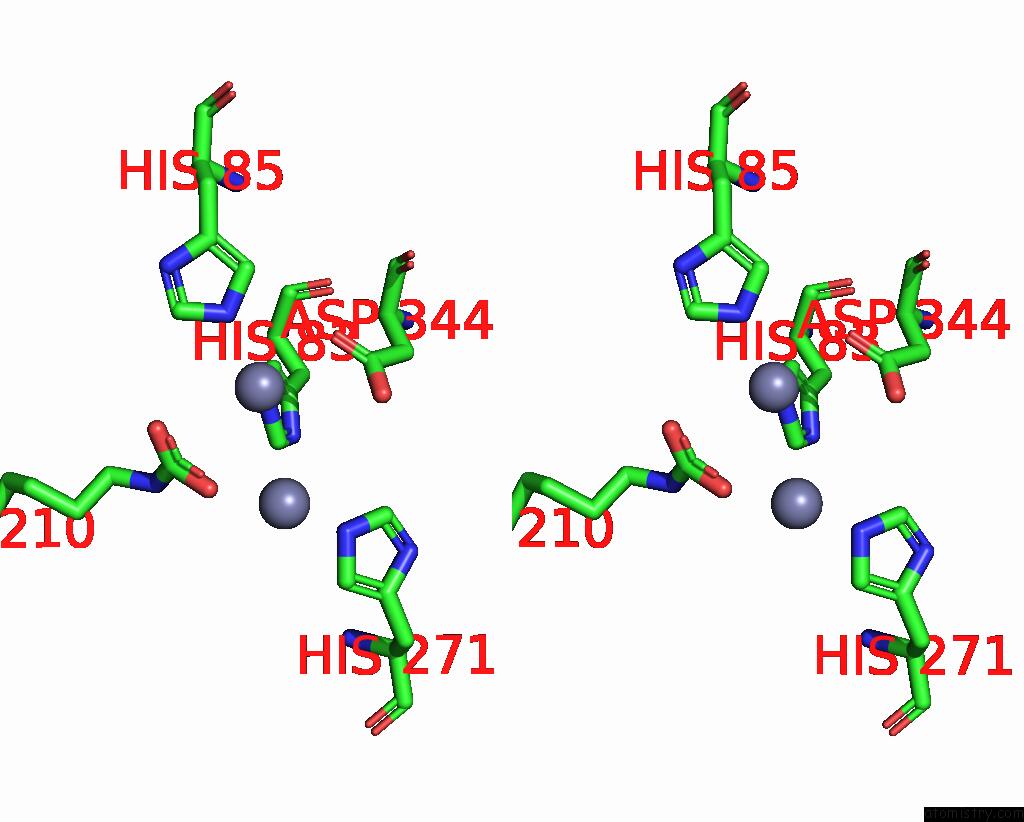
Zinc binding site 9 out of 16 in 8yag
Go back to
Zinc binding site 9 out
of 16 in the Cryo-Electron Microscopic Structure of An Amide Hydrolase From Pseudoxanthomonas Wuyuanensis
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 9 of Cryo-Electron Microscopic Structure of An Amide Hydrolase From Pseudoxanthomonas Wuyuanensis within 5.0Å range:
|
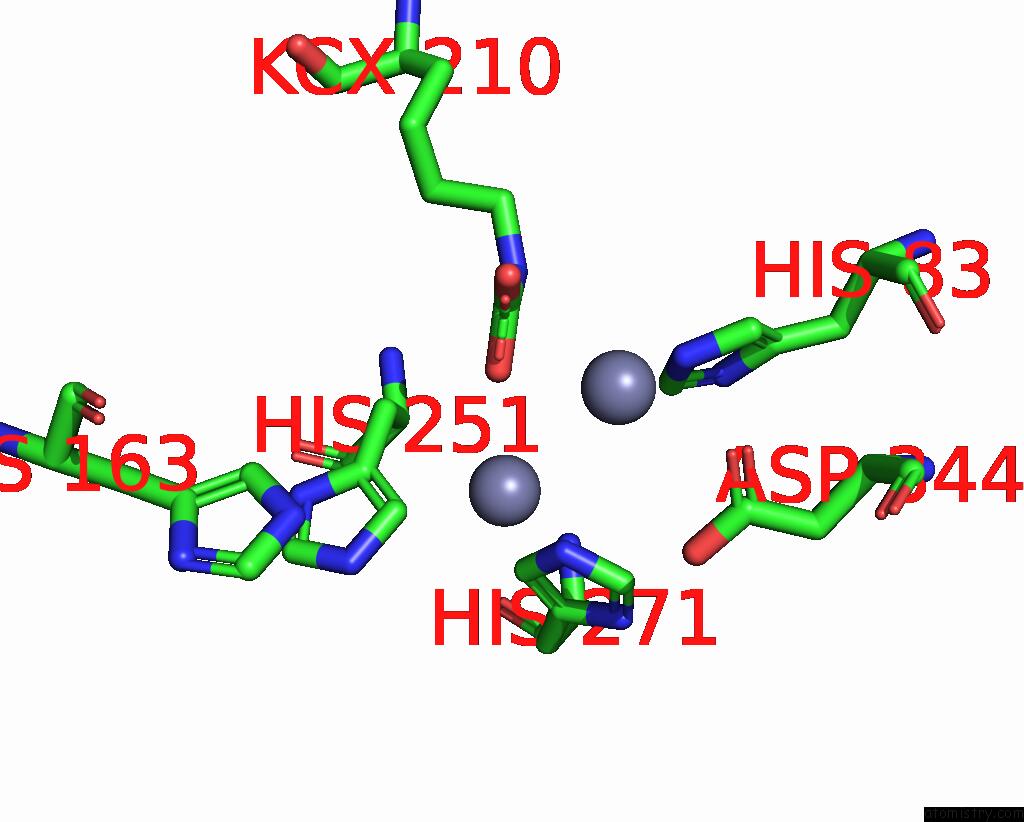
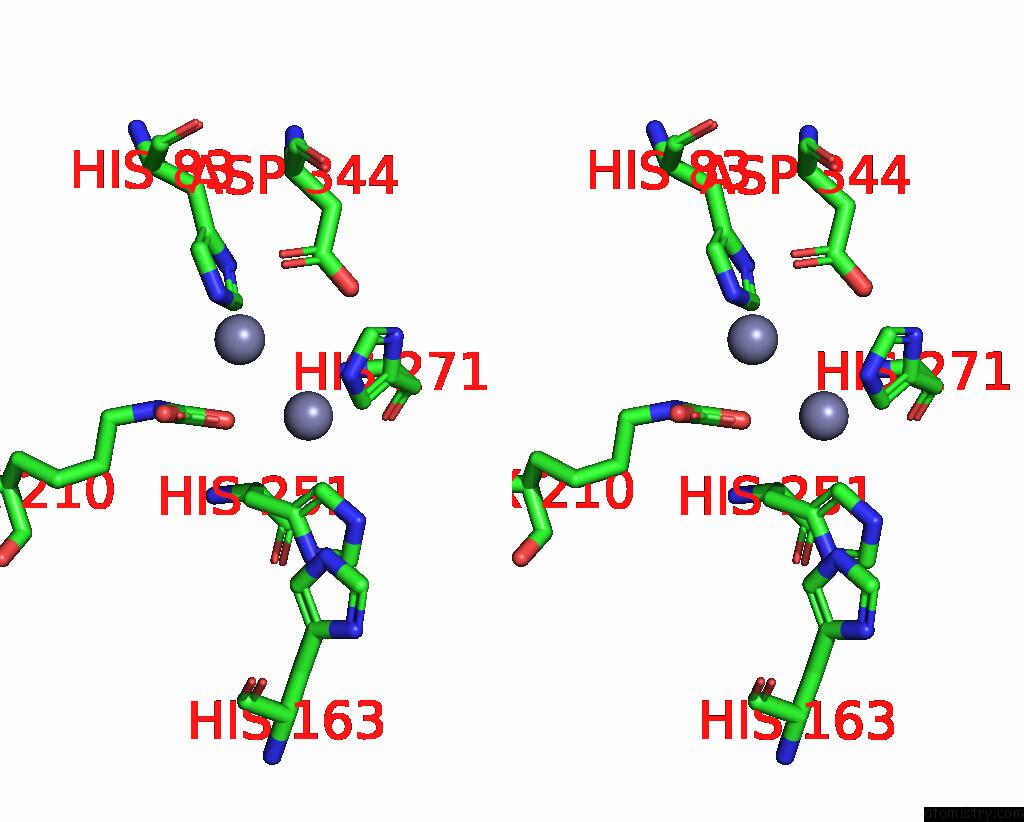
Zinc binding site 10 out of 16 in 8yag
Go back to
Zinc binding site 10 out
of 16 in the Cryo-Electron Microscopic Structure of An Amide Hydrolase From Pseudoxanthomonas Wuyuanensis
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 10 of Cryo-Electron Microscopic Structure of An Amide Hydrolase From Pseudoxanthomonas Wuyuanensis within 5.0Å range:
|
Reference:
Y.Hu,
L.Dai,
Y.Xu,
D.Niu,
X.Yang,
Z.Xie,
P.Shen,
X.Li,
H.Li,
L.Zhang,
J.Min,
R.T.Guo,
C.C.Chen.
Functional Characterization and Structural Basis of An Efficient Ochratoxin A-Degrading Amidohydrolase. Int.J.Biol.Macromol. V. 278 34831 2024.
ISSN: ISSN 0141-8130
PubMed: 39163957
DOI: 10.1016/J.IJBIOMAC.2024.134831
Page generated: Fri Aug 22 15:49:06 2025
ISSN: ISSN 0141-8130
PubMed: 39163957
DOI: 10.1016/J.IJBIOMAC.2024.134831
Last articles
Zr in 1XC1Zr in 6Y7P
Zr in 6GNL
Zr in 6HYB
Zr in 4XYY
Zr in 5KHP
Zn in 9VXG
Zn in 9VWY
Zn in 9VCL
Zn in 9VKN