Zinc »
PDB 6sdm-6sj3 »
6si2 »
Zinc in PDB 6si2: P53 Cancer Mutant Y220S
Protein crystallography data
The structure of P53 Cancer Mutant Y220S, PDB code: 6si2
was solved by
A.C.Joerger,
M.R.Bauer,
Structural Genomics Consortium (Sgc),
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 29.59 / 1.50 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 65.061, 71.216, 105.405, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 14.7 / 17.8 |
Zinc Binding Sites:
The binding sites of Zinc atom in the P53 Cancer Mutant Y220S
(pdb code 6si2). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 2 binding sites of Zinc where determined in the P53 Cancer Mutant Y220S, PDB code: 6si2:
Jump to Zinc binding site number: 1; 2;
In total 2 binding sites of Zinc where determined in the P53 Cancer Mutant Y220S, PDB code: 6si2:
Jump to Zinc binding site number: 1; 2;
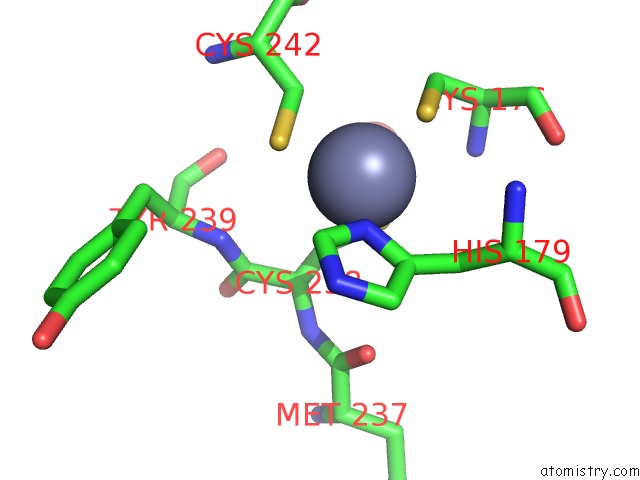
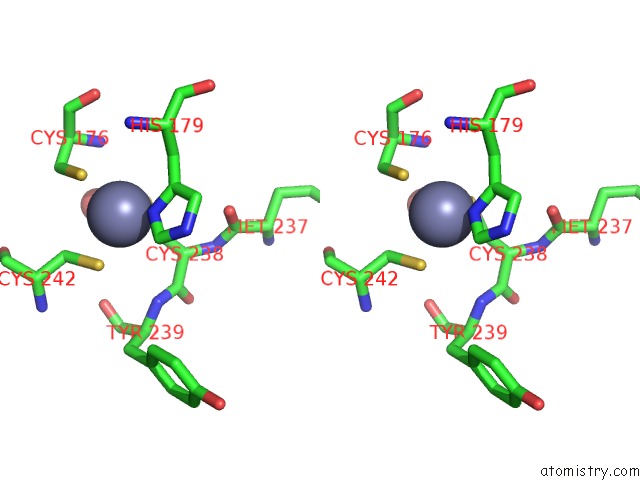
Zinc binding site 1 out of 2 in 6si2
Go back to
Zinc binding site 1 out
of 2 in the P53 Cancer Mutant Y220S
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of P53 Cancer Mutant Y220S within 5.0Å range:
|
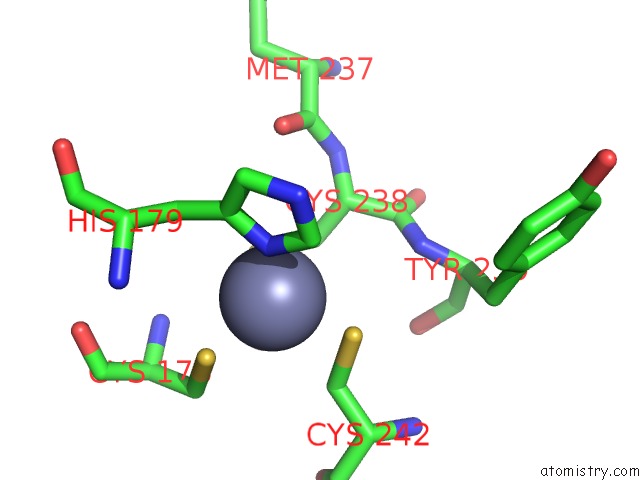
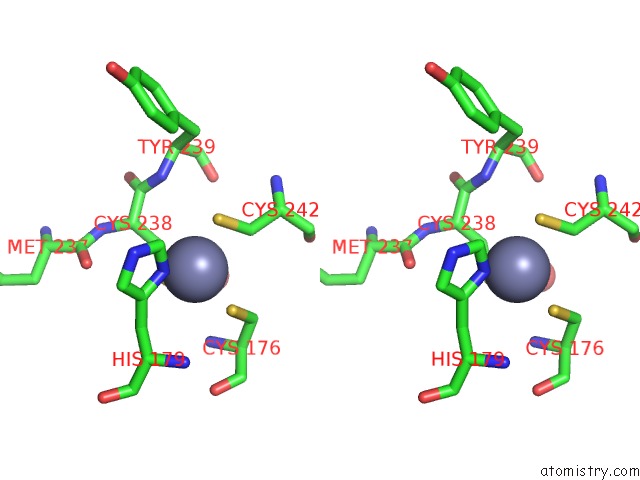
Zinc binding site 2 out of 2 in 6si2
Go back to
Zinc binding site 2 out
of 2 in the P53 Cancer Mutant Y220S
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of P53 Cancer Mutant Y220S within 5.0Å range:
|
Reference:
M.R.Bauer,
A.Kramer,
G.Settanni,
R.N.Jones,
X.Ni,
R.Khan Tareque,
A.R.Fersht,
J.Spencer,
A.C.Joerger.
Targeting Cavity-Creating P53 Cancer Mutations with Small-Molecule Stabilizers: the Y220X Paradigm. Acs Chem.Biol. 2020.
ISSN: ESSN 1554-8937
PubMed: 31990523
DOI: 10.1021/ACSCHEMBIO.9B00748
Page generated: Thu Aug 21 19:38:28 2025
ISSN: ESSN 1554-8937
PubMed: 31990523
DOI: 10.1021/ACSCHEMBIO.9B00748
Last articles
Zn in 7AUWZn in 7AUC
Zn in 7ASU
Zn in 7AT1
Zn in 7ATP
Zn in 7ARD
Zn in 7ASJ
Zn in 7ARC
Zn in 7ARU
Zn in 7ARB