Zinc »
PDB 3t73-3tg5 »
3ten »
Zinc in PDB 3ten: Holo Form of Carbon Disulfide Hydrolase
Protein crystallography data
The structure of Holo Form of Carbon Disulfide Hydrolase, PDB code: 3ten
was solved by
M.J.Smeulders,
T.R.M.B.Barends,
A.Pol,
A.Scherer,
M.H.Zandvoort,
A.Udvarhelyi,
A.Khadem,
A.Menzel,
J.Hermans,
R.L.Shoeman,
H.J.C.T.Wessels,
L.P.Van Den Heuvel,
L.Russ,
I.Schlichting,
M.S.M.Jetten,
H.J.M.Op Den Camp,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 40.00 / 2.60 |
| Space group | P 21 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 204.950, 113.240, 87.140, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 26.6 / 29.9 |
Zinc Binding Sites:
The binding sites of Zinc atom in the Holo Form of Carbon Disulfide Hydrolase
(pdb code 3ten). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 8 binding sites of Zinc where determined in the Holo Form of Carbon Disulfide Hydrolase, PDB code: 3ten:
Jump to Zinc binding site number: 1; 2; 3; 4; 5; 6; 7; 8;
In total 8 binding sites of Zinc where determined in the Holo Form of Carbon Disulfide Hydrolase, PDB code: 3ten:
Jump to Zinc binding site number: 1; 2; 3; 4; 5; 6; 7; 8;
Zinc binding site 1 out of 8 in 3ten
Go back to
Zinc binding site 1 out
of 8 in the Holo Form of Carbon Disulfide Hydrolase
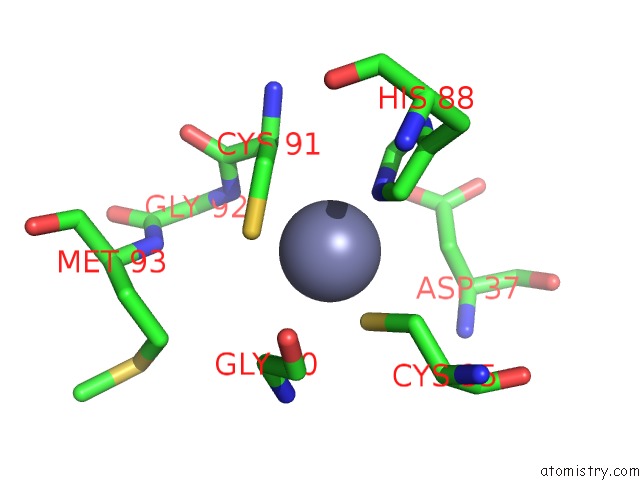
Mono view
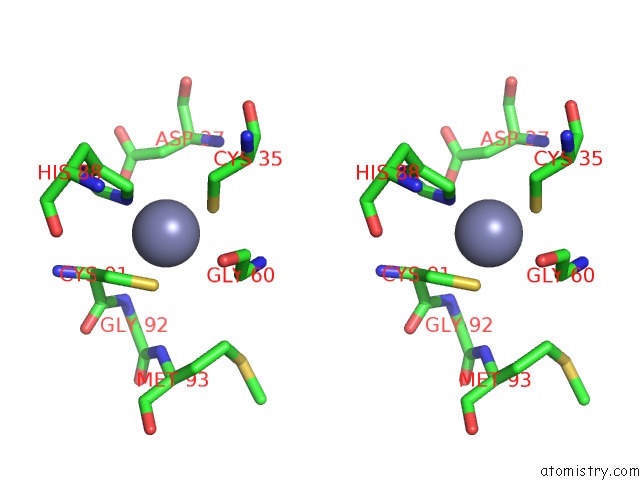
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Holo Form of Carbon Disulfide Hydrolase within 5.0Å range:
|
Zinc binding site 2 out of 8 in 3ten
Go back to
Zinc binding site 2 out
of 8 in the Holo Form of Carbon Disulfide Hydrolase
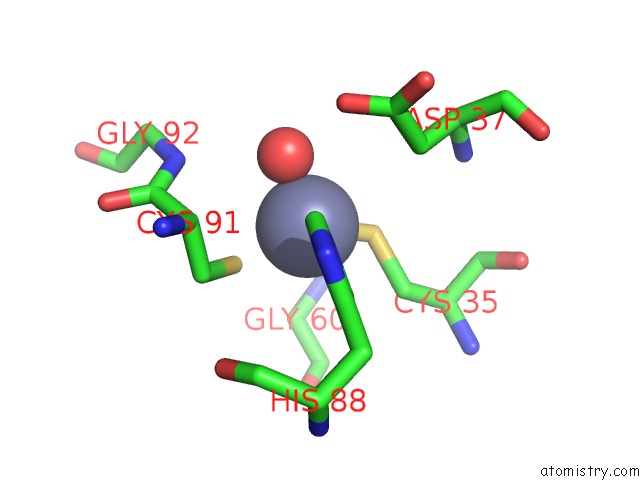
Mono view
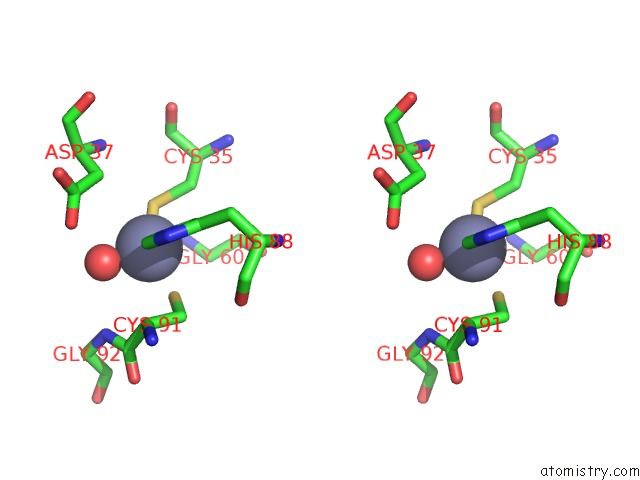
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of Holo Form of Carbon Disulfide Hydrolase within 5.0Å range:
|
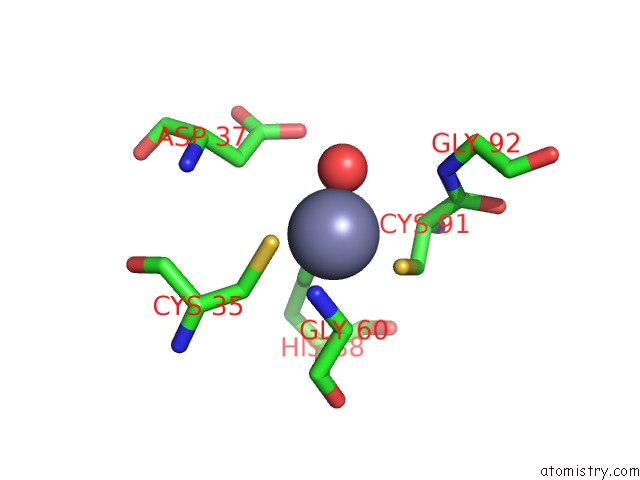
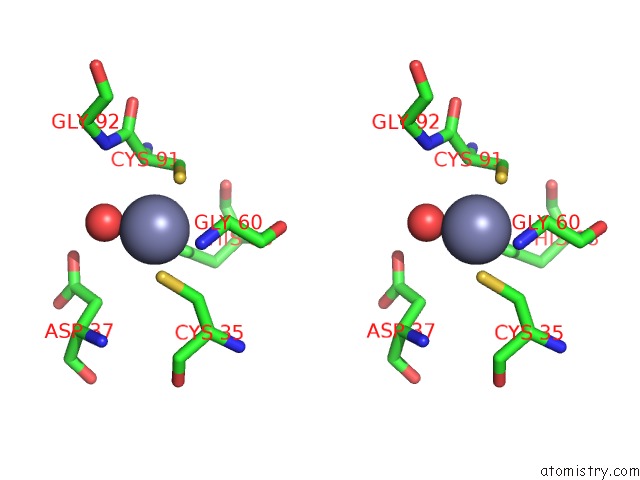
Zinc binding site 3 out of 8 in 3ten
Go back to
Zinc binding site 3 out
of 8 in the Holo Form of Carbon Disulfide Hydrolase
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 3 of Holo Form of Carbon Disulfide Hydrolase within 5.0Å range:
|
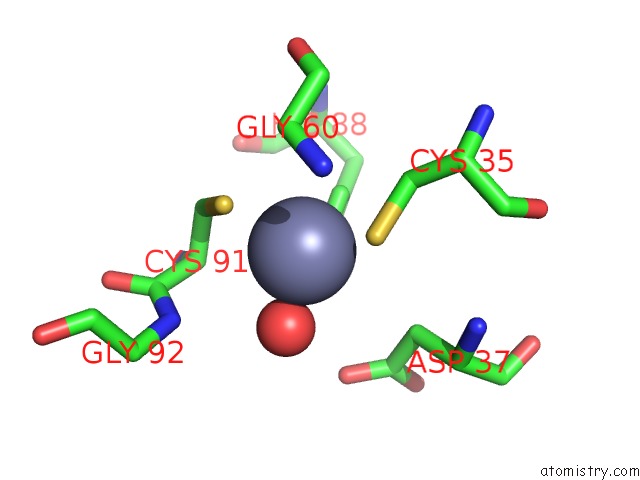
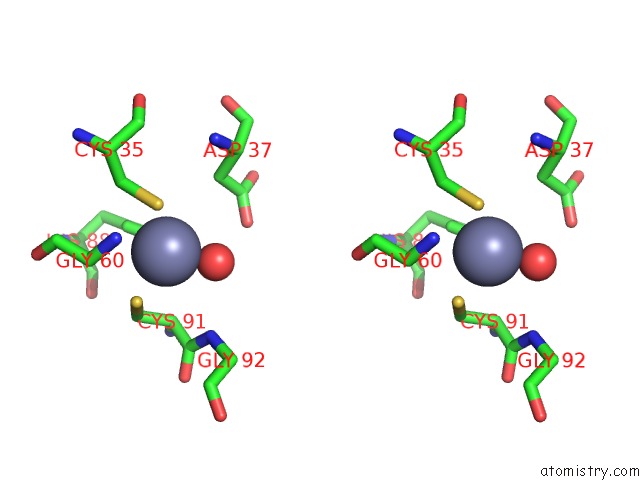
Zinc binding site 4 out of 8 in 3ten
Go back to
Zinc binding site 4 out
of 8 in the Holo Form of Carbon Disulfide Hydrolase
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 4 of Holo Form of Carbon Disulfide Hydrolase within 5.0Å range:
|
Zinc binding site 5 out of 8 in 3ten
Go back to
Zinc binding site 5 out
of 8 in the Holo Form of Carbon Disulfide Hydrolase
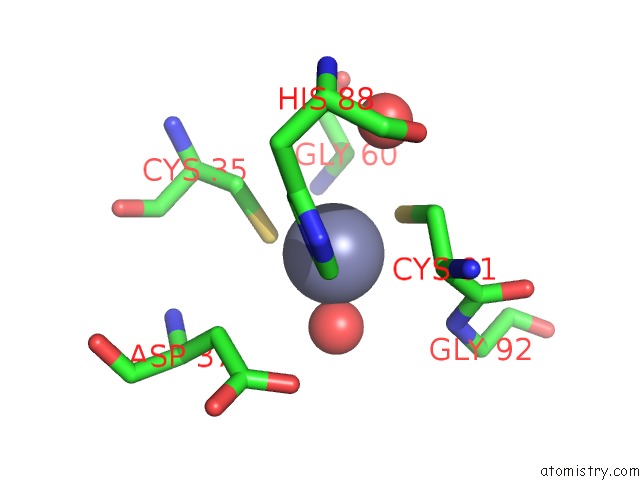
Mono view
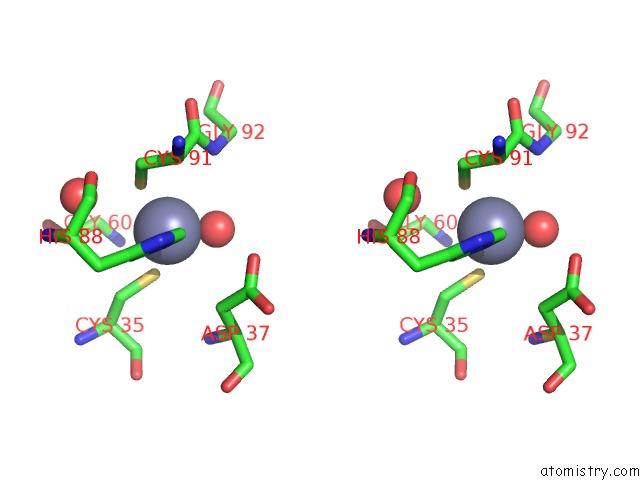
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 5 of Holo Form of Carbon Disulfide Hydrolase within 5.0Å range:
|
Zinc binding site 6 out of 8 in 3ten
Go back to
Zinc binding site 6 out
of 8 in the Holo Form of Carbon Disulfide Hydrolase
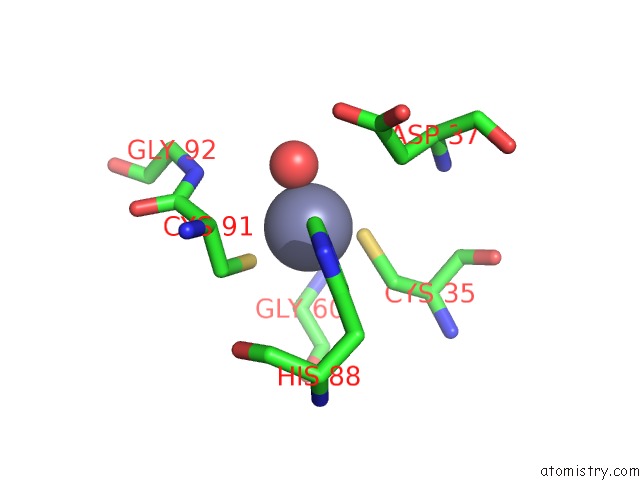
Mono view
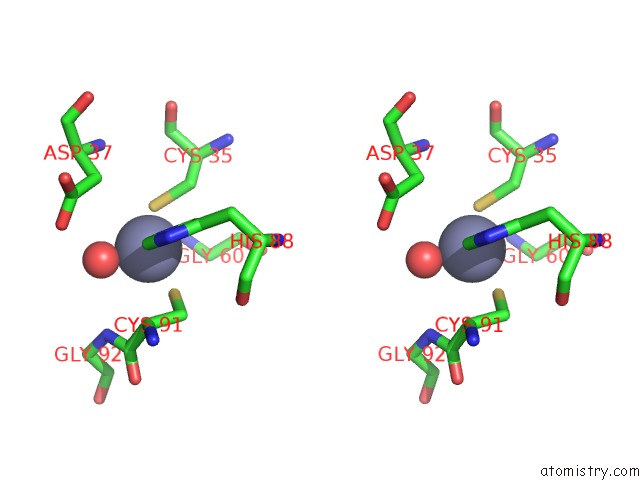
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 6 of Holo Form of Carbon Disulfide Hydrolase within 5.0Å range:
|
Zinc binding site 7 out of 8 in 3ten
Go back to
Zinc binding site 7 out
of 8 in the Holo Form of Carbon Disulfide Hydrolase
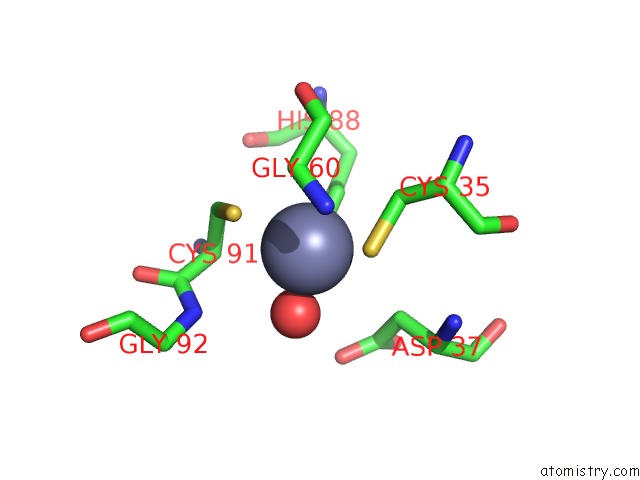
Mono view
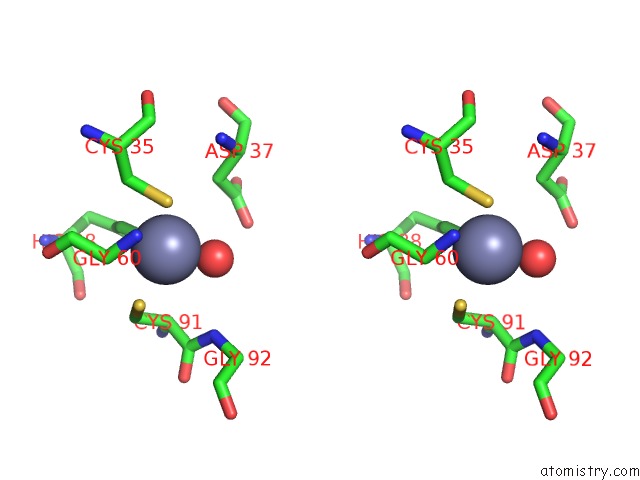
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 7 of Holo Form of Carbon Disulfide Hydrolase within 5.0Å range:
|
Zinc binding site 8 out of 8 in 3ten
Go back to
Zinc binding site 8 out
of 8 in the Holo Form of Carbon Disulfide Hydrolase
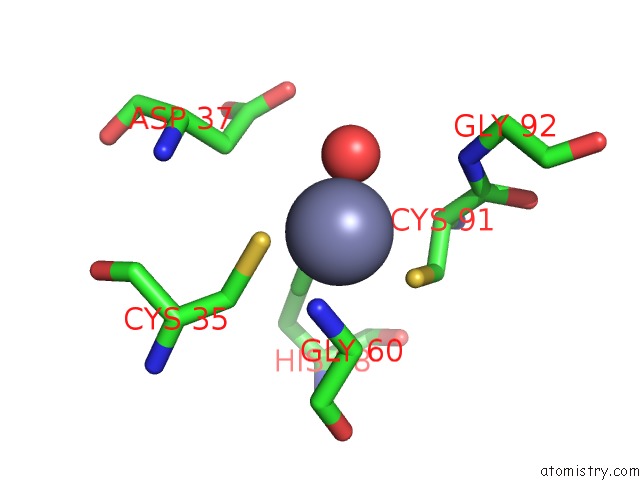
Mono view
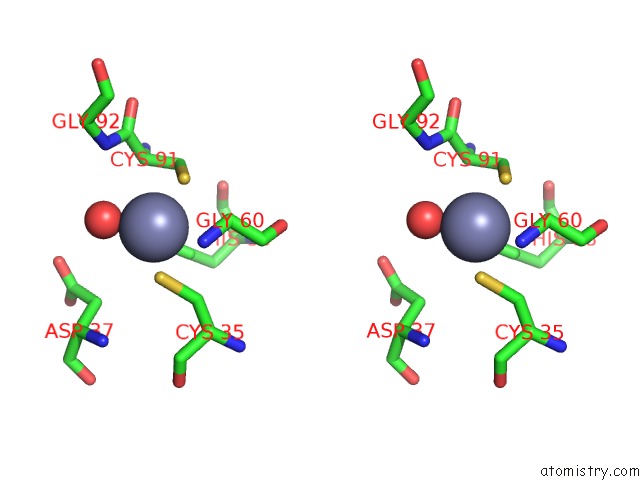
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 8 of Holo Form of Carbon Disulfide Hydrolase within 5.0Å range:
|
Reference:
M.J.Smeulders,
T.R.Barends,
A.Pol,
A.Scherer,
M.H.Zandvoort,
A.Udvarhelyi,
A.F.Khadem,
A.Menzel,
J.Hermans,
R.L.Shoeman,
H.J.Wessels,
L.P.Van Den Heuvel,
L.Russ,
I.Schlichting,
M.S.Jetten,
H.J.Op Den Camp.
Evolution of A New Enzyme For Carbon Disulphide Conversion By An Acidothermophilic Archaeon. Nature V. 478 412 2011.
ISSN: ISSN 0028-0836
PubMed: 22012399
DOI: 10.1038/NATURE10464
Page generated: Wed Aug 20 14:27:09 2025
ISSN: ISSN 0028-0836
PubMed: 22012399
DOI: 10.1038/NATURE10464
Last articles
Zn in 4KYHZn in 4KXQ
Zn in 4KXD
Zn in 4KXB
Zn in 4KXC
Zn in 4KXA
Zn in 4KW9
Zn in 4KVP
Zn in 4KX9
Zn in 4KX8