Zinc »
PDB 8y9c-8z0p »
8yak »
Zinc in PDB 8yak: Cryo-Em Structure and Rational Engineering of A Novel Efficient Ochratoxin A-Detoxifying Amidohydrolase
Other elements in 8yak:
The structure of Cryo-Em Structure and Rational Engineering of A Novel Efficient Ochratoxin A-Detoxifying Amidohydrolase also contains other interesting chemical elements:
| Chlorine | (Cl) | 8 atoms |
Zinc Binding Sites:
Pages:
>>> Page 1 <<< Page 2, Binding sites: 11 - 16;Binding sites:
The binding sites of Zinc atom in the Cryo-Em Structure and Rational Engineering of A Novel Efficient Ochratoxin A-Detoxifying Amidohydrolase (pdb code 8yak). This binding sites where shown within 5.0 Angstroms radius around Zinc atom.In total 16 binding sites of Zinc where determined in the Cryo-Em Structure and Rational Engineering of A Novel Efficient Ochratoxin A-Detoxifying Amidohydrolase, PDB code: 8yak:
Jump to Zinc binding site number: 1; 2; 3; 4; 5; 6; 7; 8; 9; 10;
Zinc binding site 1 out of 16 in 8yak
Go back to
Zinc binding site 1 out
of 16 in the Cryo-Em Structure and Rational Engineering of A Novel Efficient Ochratoxin A-Detoxifying Amidohydrolase
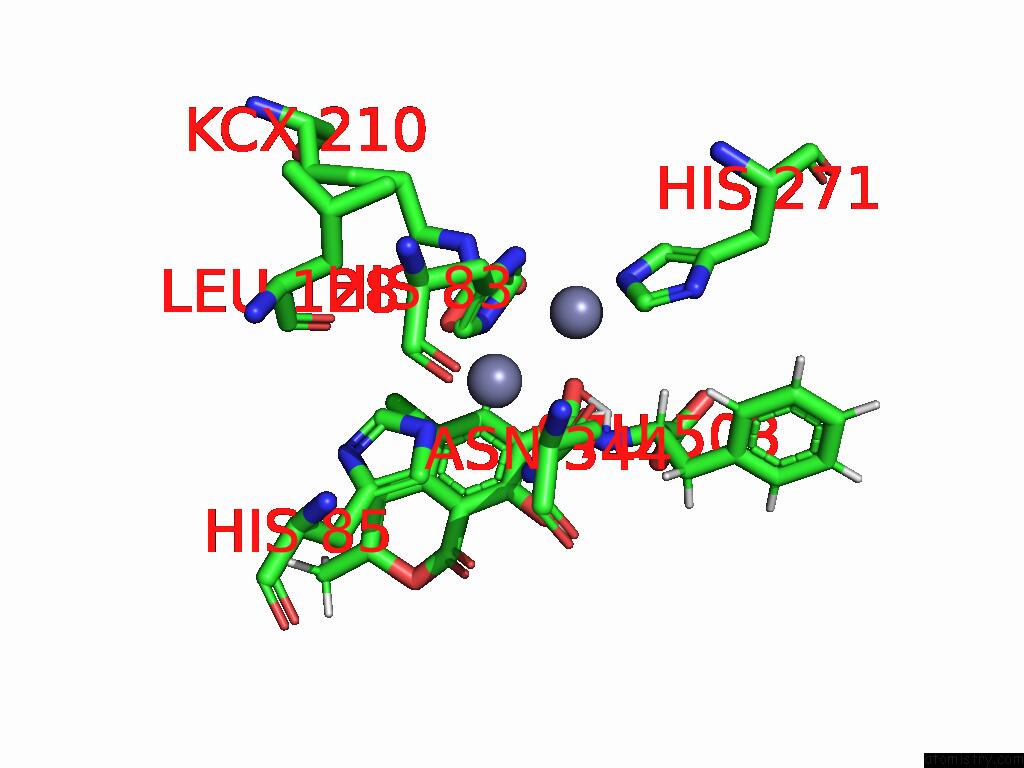
Mono view
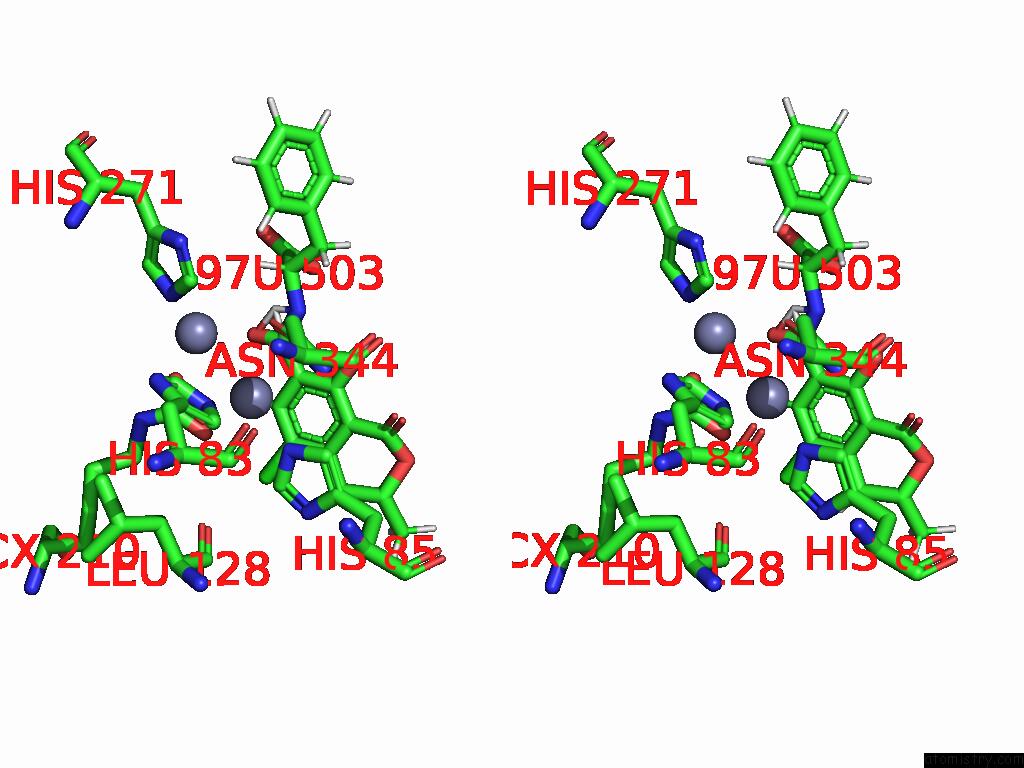
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Cryo-Em Structure and Rational Engineering of A Novel Efficient Ochratoxin A-Detoxifying Amidohydrolase within 5.0Å range:
|
Zinc binding site 2 out of 16 in 8yak
Go back to
Zinc binding site 2 out
of 16 in the Cryo-Em Structure and Rational Engineering of A Novel Efficient Ochratoxin A-Detoxifying Amidohydrolase
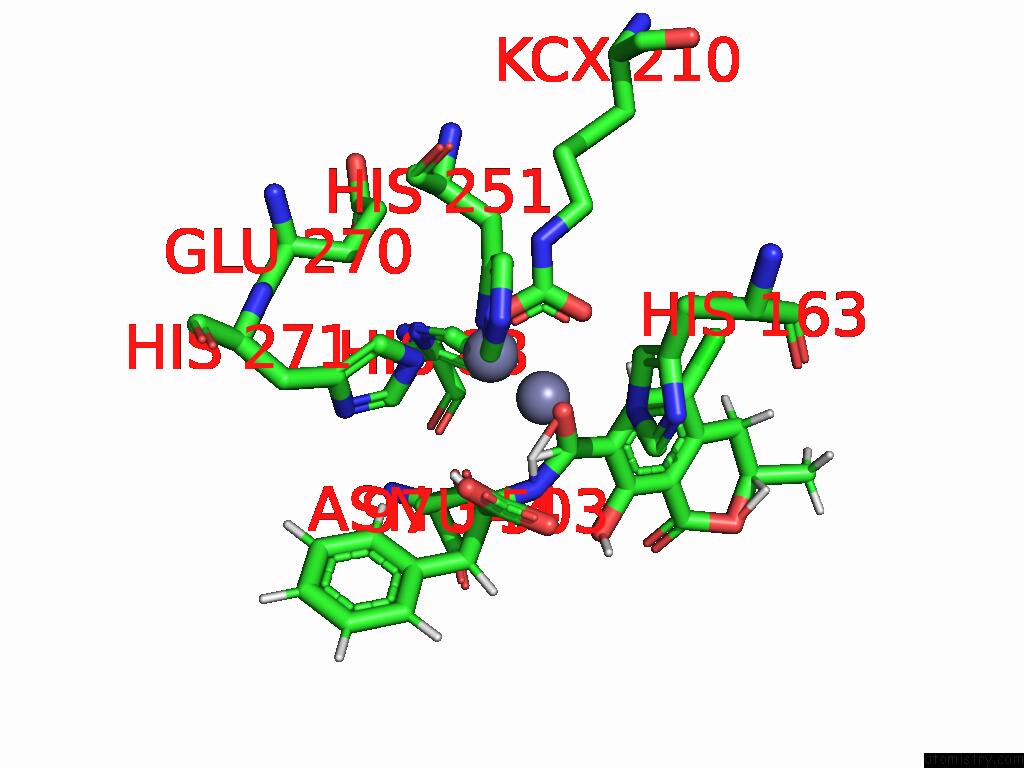
Mono view
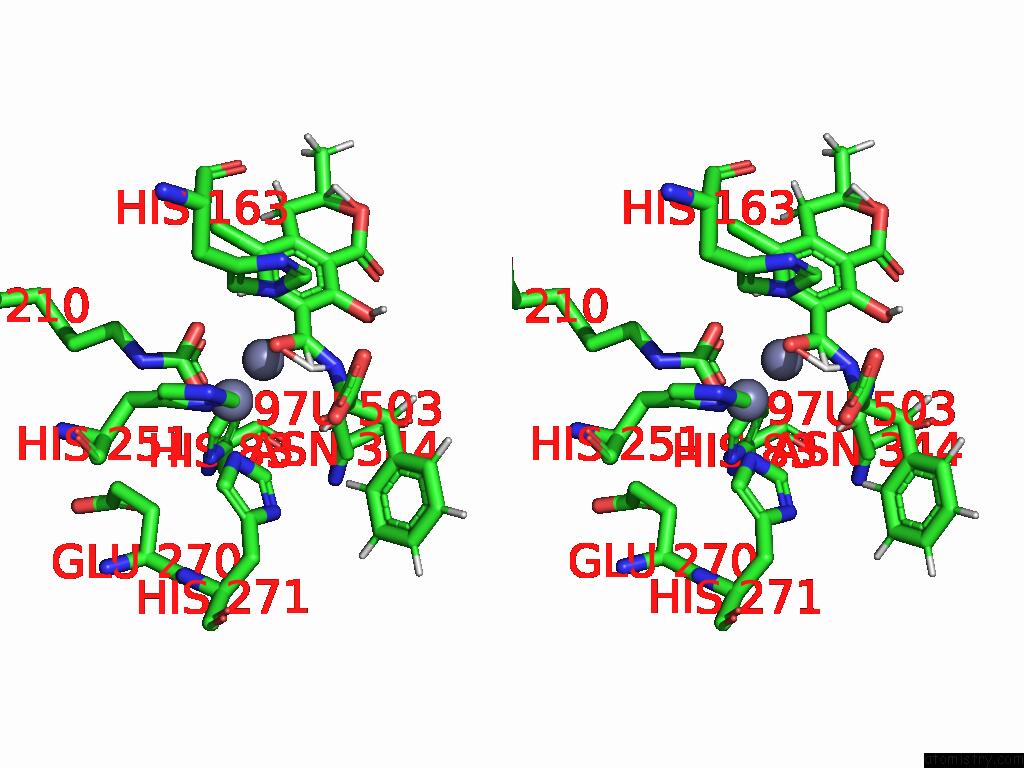
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of Cryo-Em Structure and Rational Engineering of A Novel Efficient Ochratoxin A-Detoxifying Amidohydrolase within 5.0Å range:
|
Zinc binding site 3 out of 16 in 8yak
Go back to
Zinc binding site 3 out
of 16 in the Cryo-Em Structure and Rational Engineering of A Novel Efficient Ochratoxin A-Detoxifying Amidohydrolase
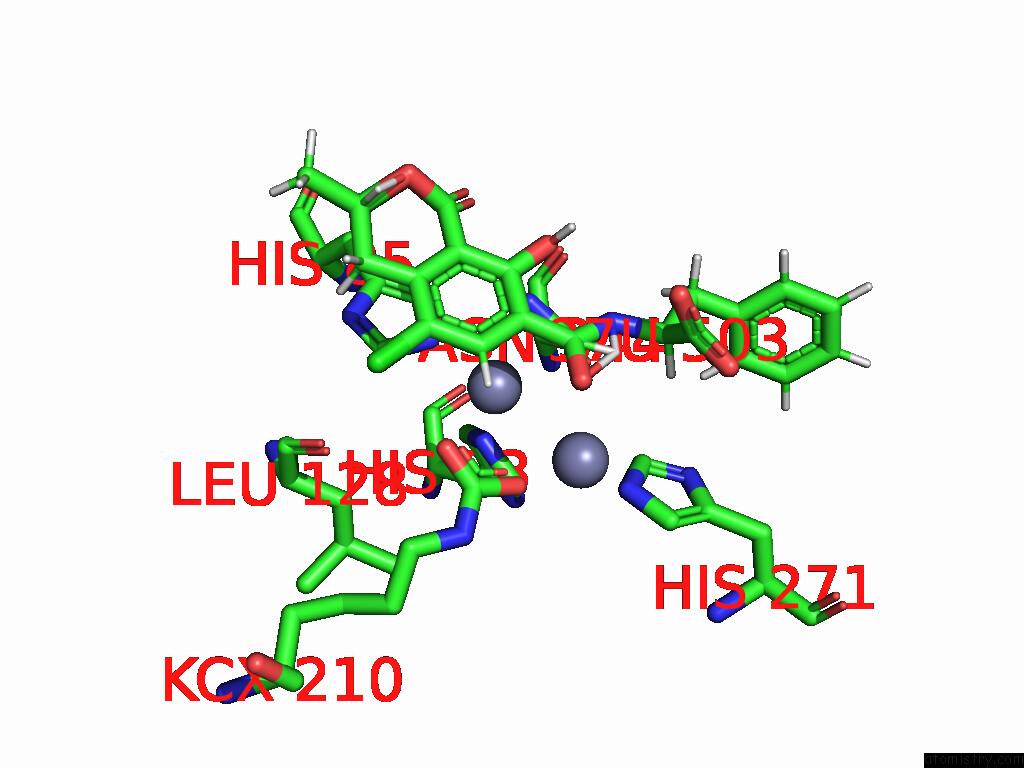
Mono view
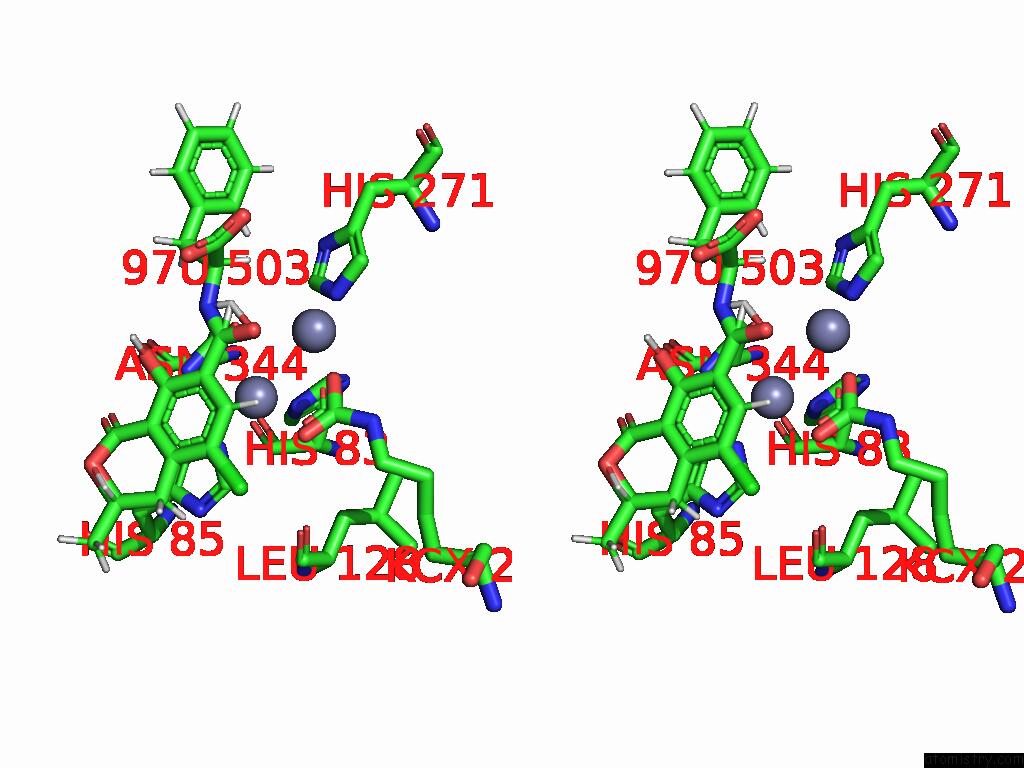
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 3 of Cryo-Em Structure and Rational Engineering of A Novel Efficient Ochratoxin A-Detoxifying Amidohydrolase within 5.0Å range:
|
Zinc binding site 4 out of 16 in 8yak
Go back to
Zinc binding site 4 out
of 16 in the Cryo-Em Structure and Rational Engineering of A Novel Efficient Ochratoxin A-Detoxifying Amidohydrolase
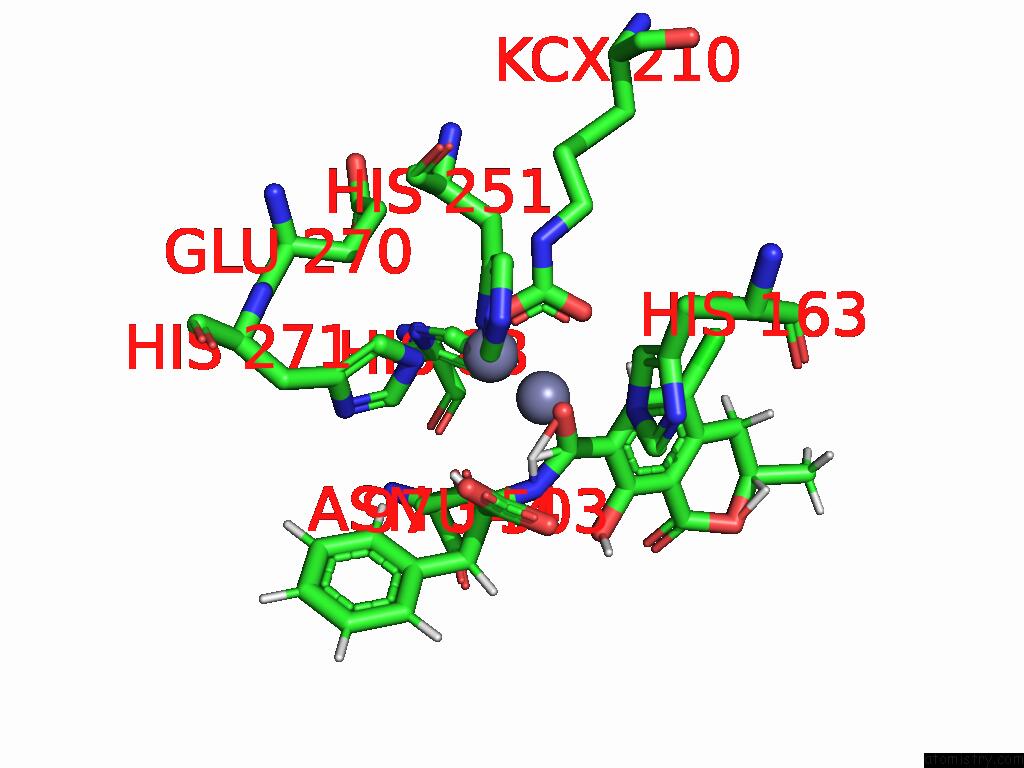
Mono view
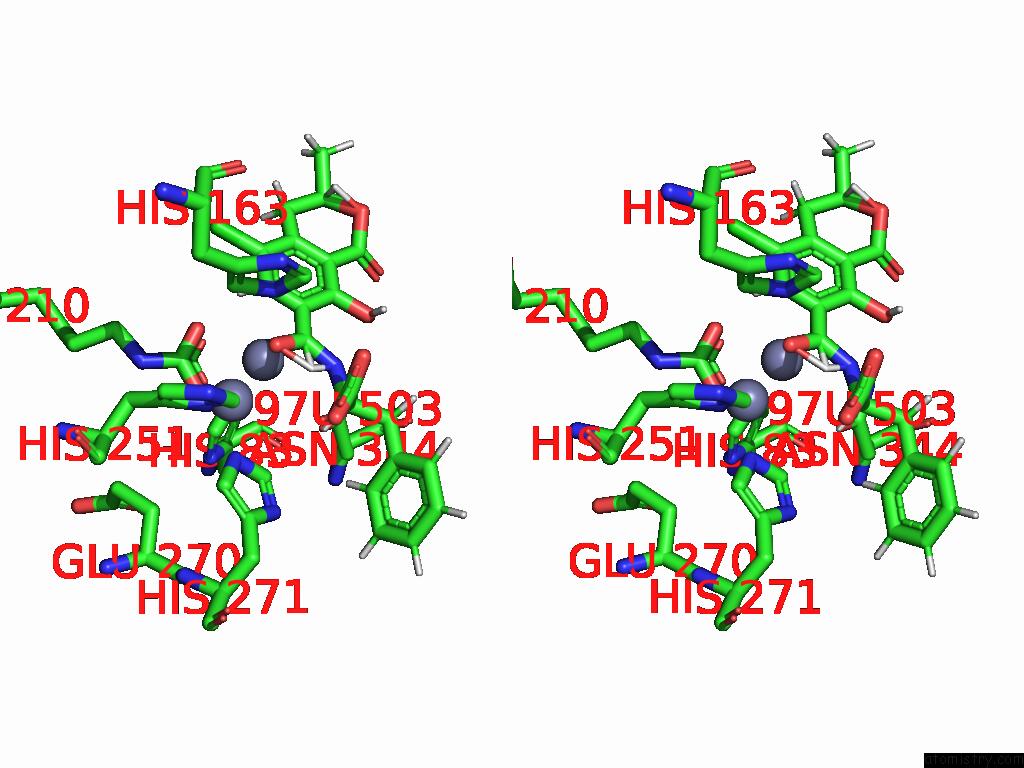
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 4 of Cryo-Em Structure and Rational Engineering of A Novel Efficient Ochratoxin A-Detoxifying Amidohydrolase within 5.0Å range:
|
Zinc binding site 5 out of 16 in 8yak
Go back to
Zinc binding site 5 out
of 16 in the Cryo-Em Structure and Rational Engineering of A Novel Efficient Ochratoxin A-Detoxifying Amidohydrolase
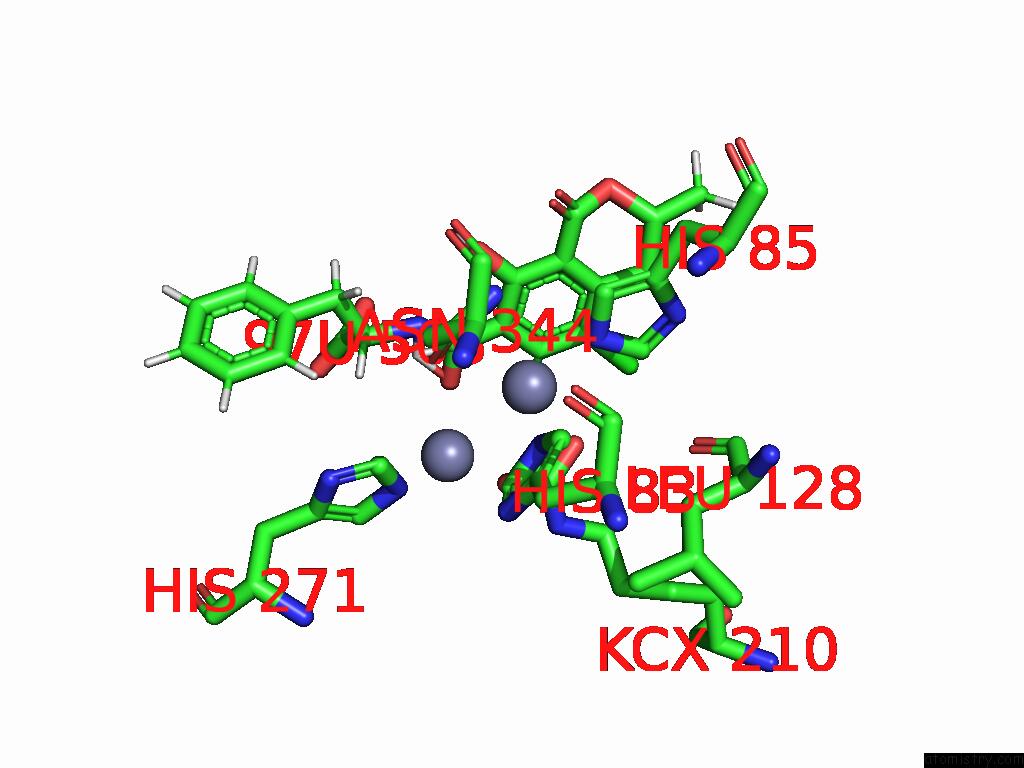
Mono view
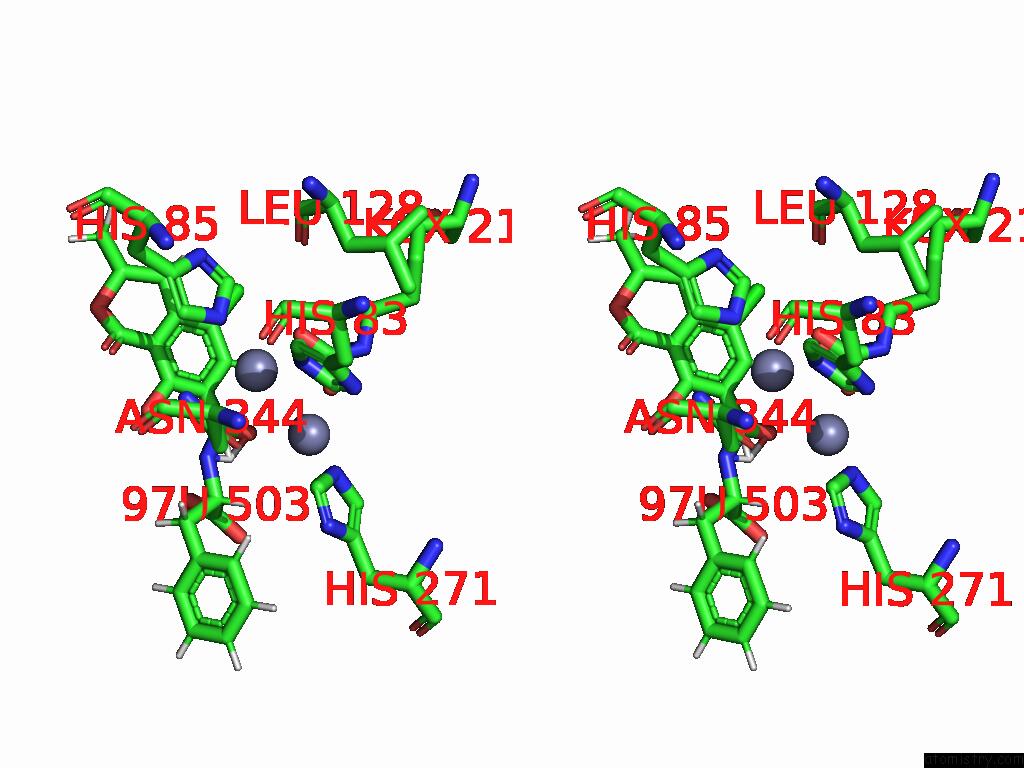
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 5 of Cryo-Em Structure and Rational Engineering of A Novel Efficient Ochratoxin A-Detoxifying Amidohydrolase within 5.0Å range:
|
Zinc binding site 6 out of 16 in 8yak
Go back to
Zinc binding site 6 out
of 16 in the Cryo-Em Structure and Rational Engineering of A Novel Efficient Ochratoxin A-Detoxifying Amidohydrolase
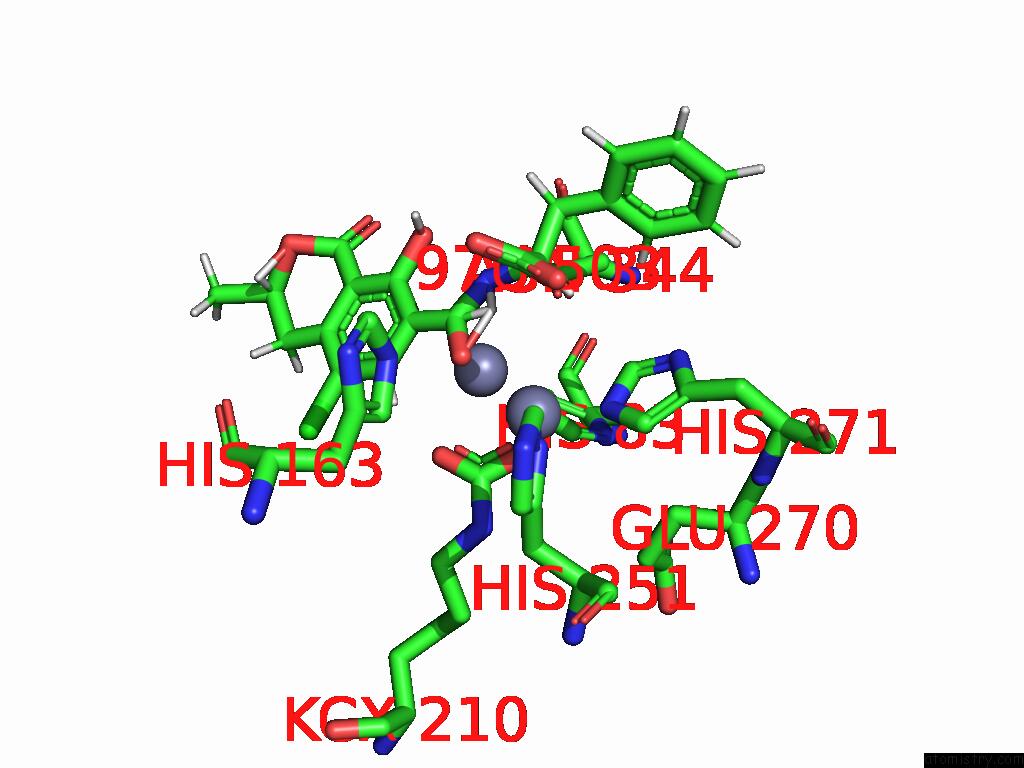
Mono view
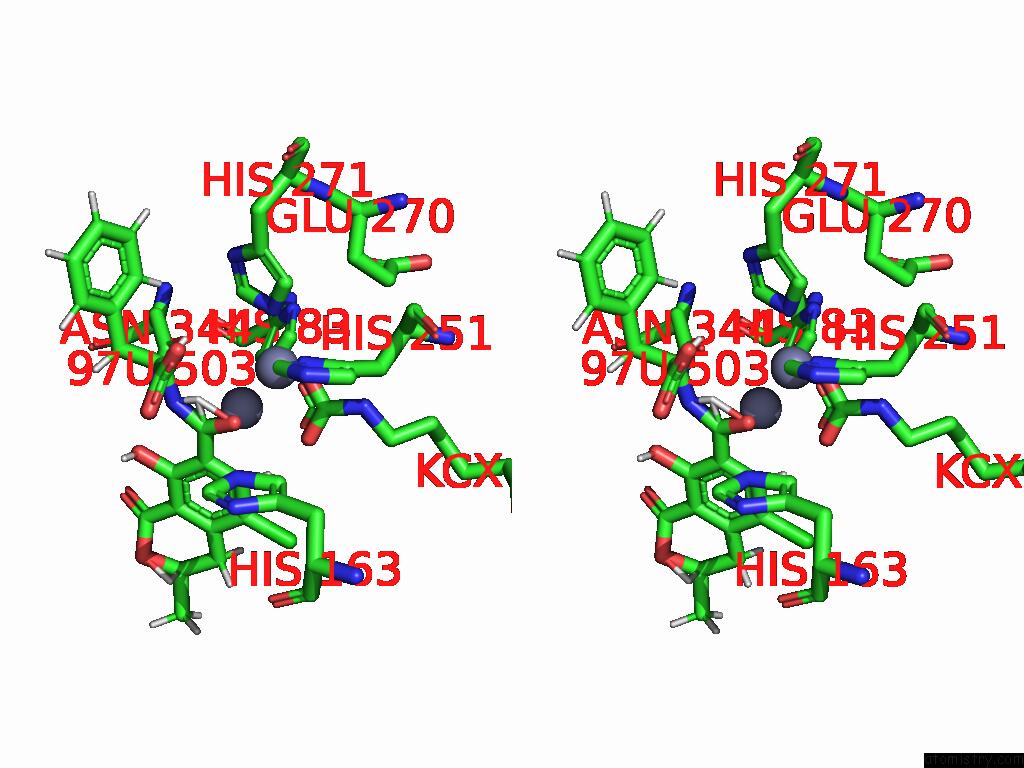
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 6 of Cryo-Em Structure and Rational Engineering of A Novel Efficient Ochratoxin A-Detoxifying Amidohydrolase within 5.0Å range:
|
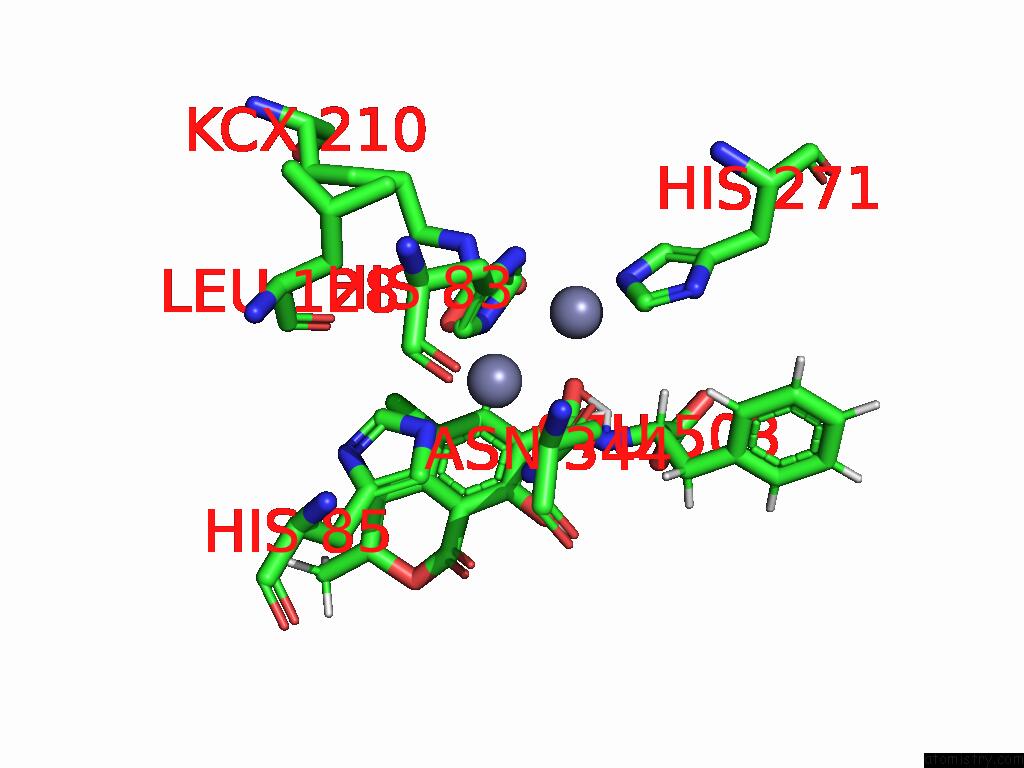
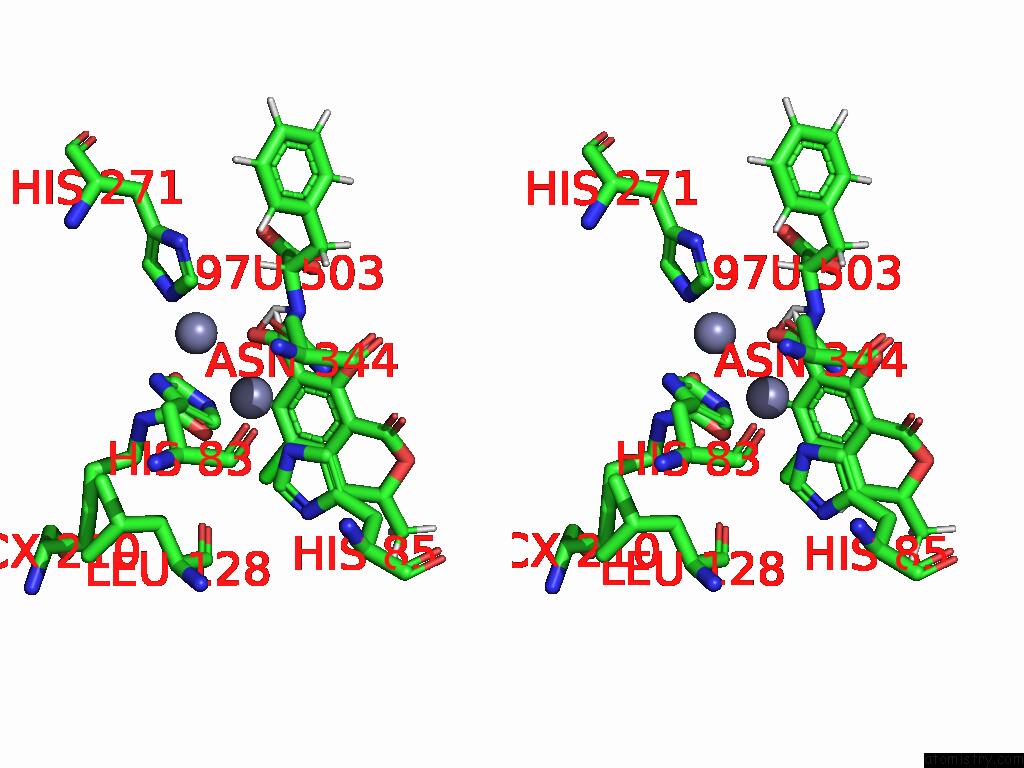
Zinc binding site 7 out of 16 in 8yak
Go back to
Zinc binding site 7 out
of 16 in the Cryo-Em Structure and Rational Engineering of A Novel Efficient Ochratoxin A-Detoxifying Amidohydrolase
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 7 of Cryo-Em Structure and Rational Engineering of A Novel Efficient Ochratoxin A-Detoxifying Amidohydrolase within 5.0Å range:
|
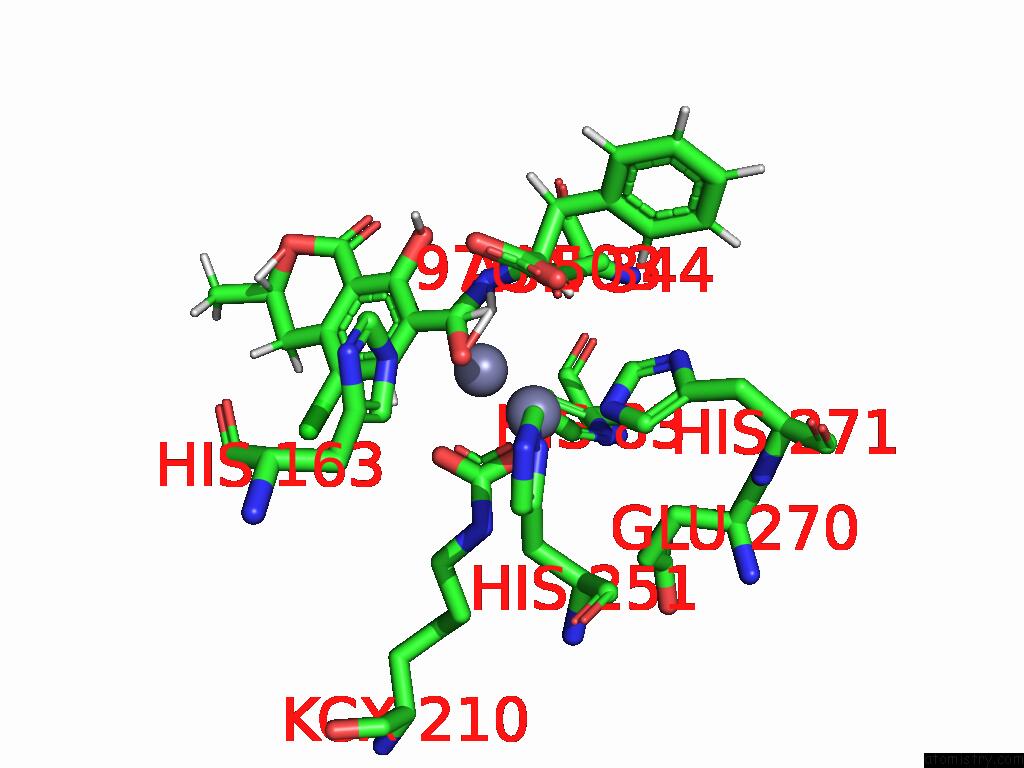
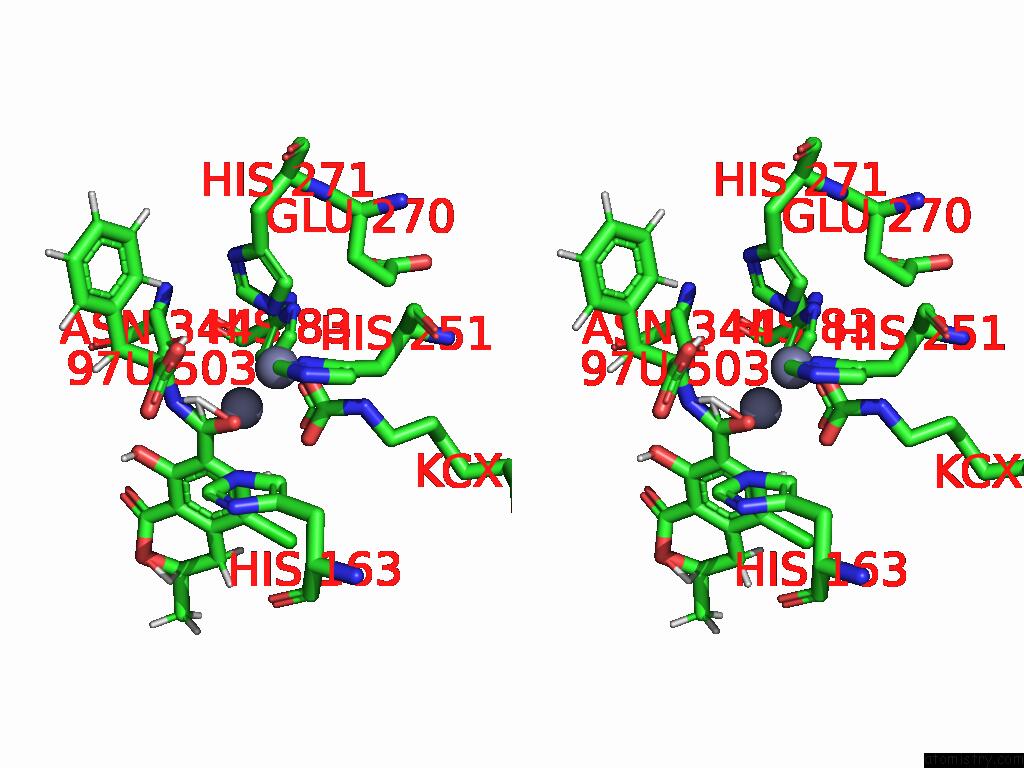
Zinc binding site 8 out of 16 in 8yak
Go back to
Zinc binding site 8 out
of 16 in the Cryo-Em Structure and Rational Engineering of A Novel Efficient Ochratoxin A-Detoxifying Amidohydrolase
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 8 of Cryo-Em Structure and Rational Engineering of A Novel Efficient Ochratoxin A-Detoxifying Amidohydrolase within 5.0Å range:
|
Zinc binding site 9 out of 16 in 8yak
Go back to
Zinc binding site 9 out
of 16 in the Cryo-Em Structure and Rational Engineering of A Novel Efficient Ochratoxin A-Detoxifying Amidohydrolase
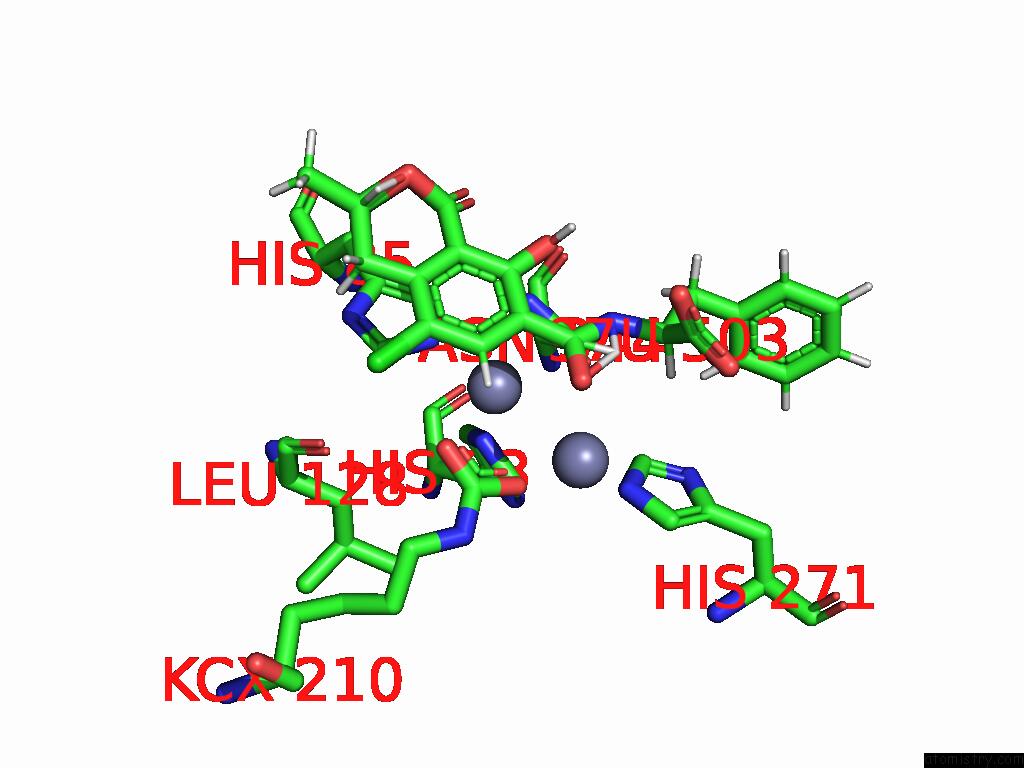
Mono view
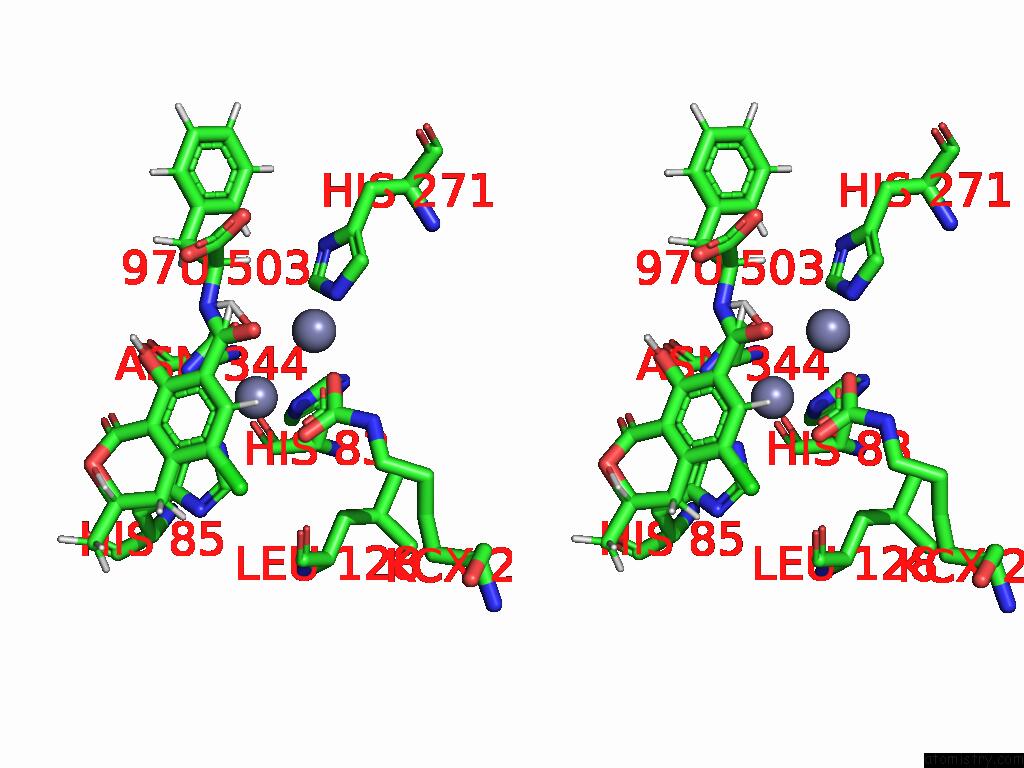
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 9 of Cryo-Em Structure and Rational Engineering of A Novel Efficient Ochratoxin A-Detoxifying Amidohydrolase within 5.0Å range:
|
Zinc binding site 10 out of 16 in 8yak
Go back to
Zinc binding site 10 out
of 16 in the Cryo-Em Structure and Rational Engineering of A Novel Efficient Ochratoxin A-Detoxifying Amidohydrolase
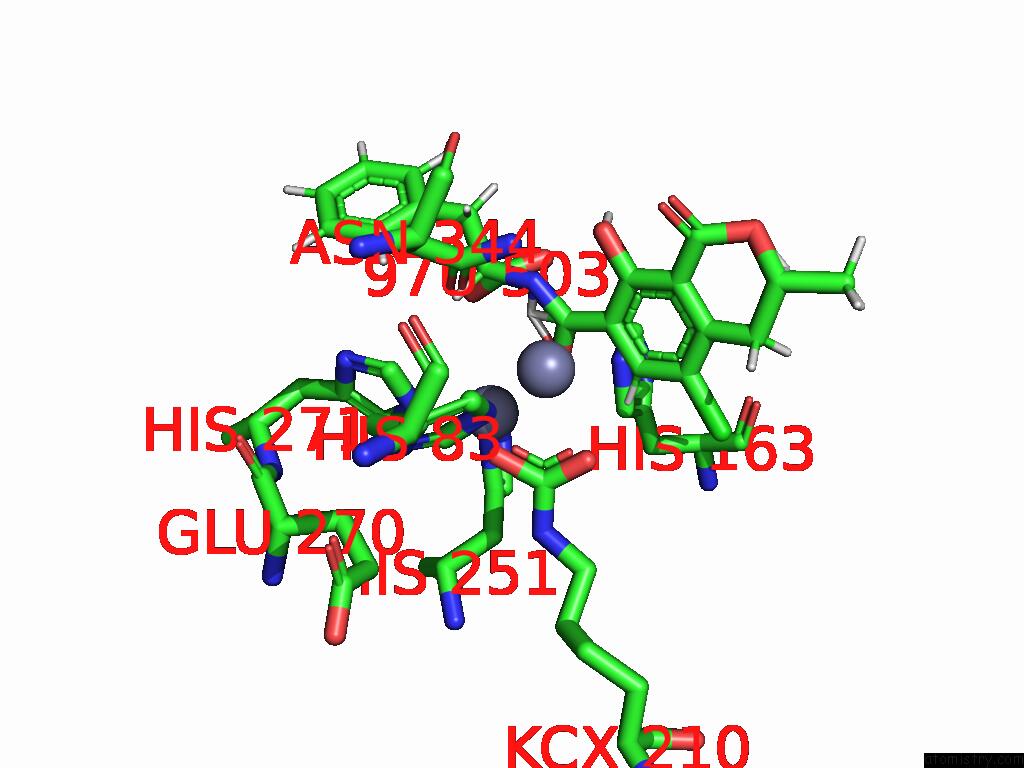
Mono view
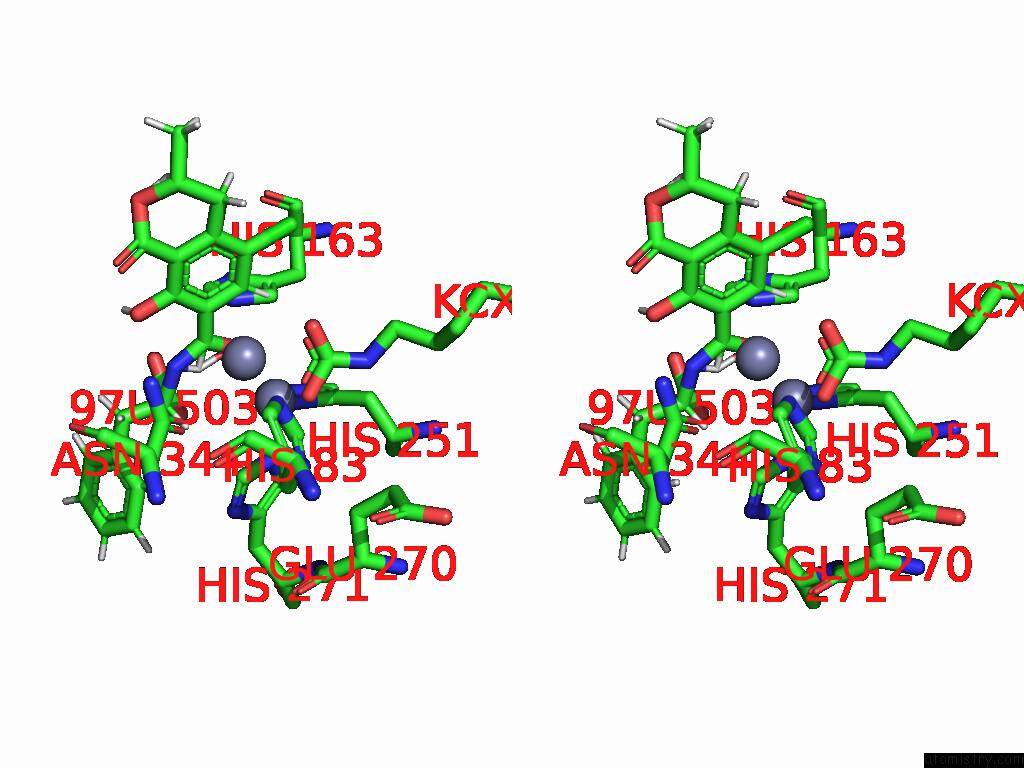
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 10 of Cryo-Em Structure and Rational Engineering of A Novel Efficient Ochratoxin A-Detoxifying Amidohydrolase within 5.0Å range:
|
Reference:
Y.Hu,
L.Dai,
Y.Xu,
D.Niu,
X.Yang,
Z.Xie,
P.Shen,
X.Li,
H.Li,
L.Zhang,
J.Min,
R.T.Guo,
C.C.Chen.
Functional Characterization and Structural Basis of An Efficient Ochratoxin A-Degrading Amidohydrolase. Int.J.Biol.Macromol. V. 278 34831 2024.
ISSN: ISSN 0141-8130
PubMed: 39163957
DOI: 10.1016/J.IJBIOMAC.2024.134831
Page generated: Sun Feb 9 00:56:46 2025
ISSN: ISSN 0141-8130
PubMed: 39163957
DOI: 10.1016/J.IJBIOMAC.2024.134831
Last articles
Zn in 9IRQZn in 9IYX
Zn in 9J8P
Zn in 9IUU
Zn in 9GBF
Zn in 9G2V
Zn in 9G2L
Zn in 9G2X
Zn in 9G2Z
Zn in 9G2K