Zinc »
PDB 8pgj-8pp6 »
8pim »
Zinc in PDB 8pim: Fully Recruited Rfah Bound to E. Coli Transcription Complex Paused at Ops Site (Not Complementary Scaffold)
Enzymatic activity of Fully Recruited Rfah Bound to E. Coli Transcription Complex Paused at Ops Site (Not Complementary Scaffold)
All present enzymatic activity of Fully Recruited Rfah Bound to E. Coli Transcription Complex Paused at Ops Site (Not Complementary Scaffold):
2.7.7.6;
2.7.7.6;
Other elements in 8pim:
The structure of Fully Recruited Rfah Bound to E. Coli Transcription Complex Paused at Ops Site (Not Complementary Scaffold) also contains other interesting chemical elements:
| Magnesium | (Mg) | 1 atom |
Zinc Binding Sites:
The binding sites of Zinc atom in the Fully Recruited Rfah Bound to E. Coli Transcription Complex Paused at Ops Site (Not Complementary Scaffold)
(pdb code 8pim). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 2 binding sites of Zinc where determined in the Fully Recruited Rfah Bound to E. Coli Transcription Complex Paused at Ops Site (Not Complementary Scaffold), PDB code: 8pim:
Jump to Zinc binding site number: 1; 2;
In total 2 binding sites of Zinc where determined in the Fully Recruited Rfah Bound to E. Coli Transcription Complex Paused at Ops Site (Not Complementary Scaffold), PDB code: 8pim:
Jump to Zinc binding site number: 1; 2;
Zinc binding site 1 out of 2 in 8pim
Go back to
Zinc binding site 1 out
of 2 in the Fully Recruited Rfah Bound to E. Coli Transcription Complex Paused at Ops Site (Not Complementary Scaffold)
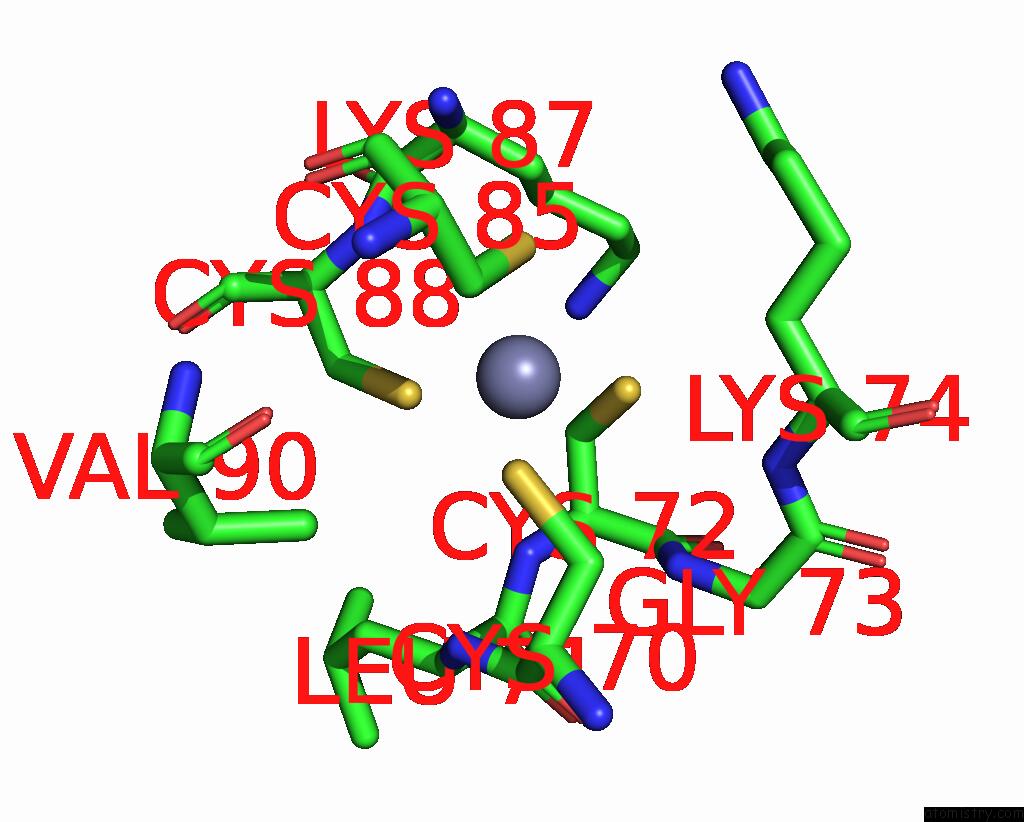
Mono view
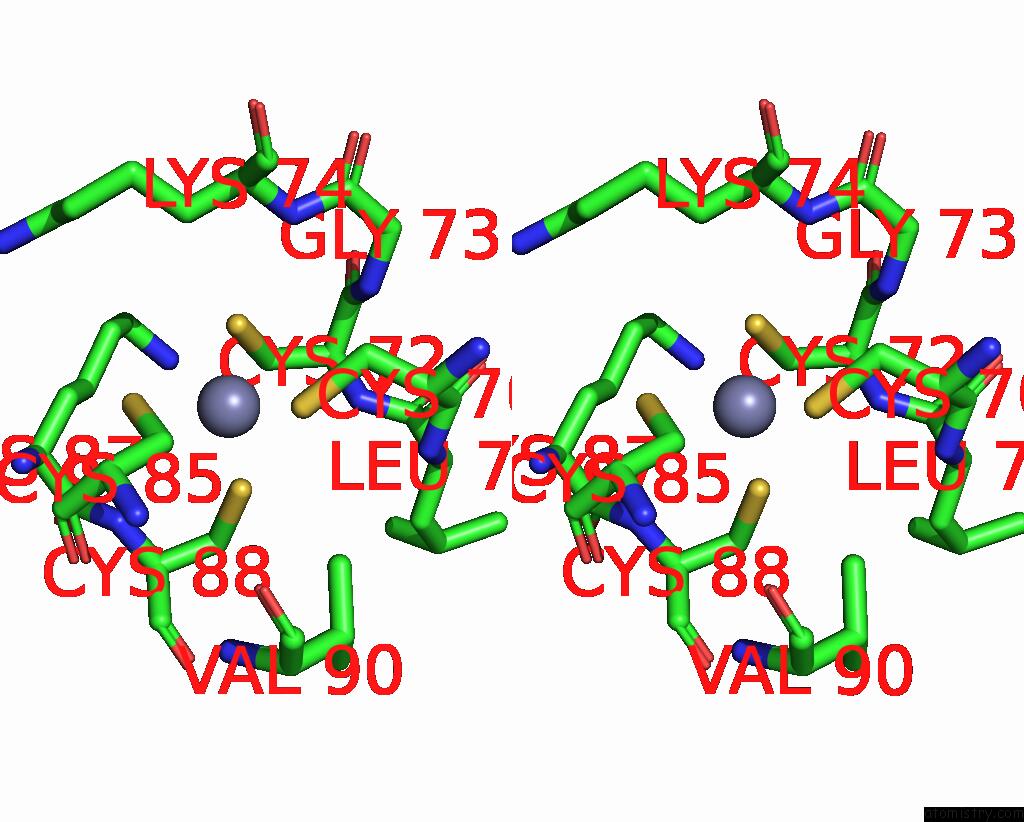
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Fully Recruited Rfah Bound to E. Coli Transcription Complex Paused at Ops Site (Not Complementary Scaffold) within 5.0Å range:
|
Zinc binding site 2 out of 2 in 8pim
Go back to
Zinc binding site 2 out
of 2 in the Fully Recruited Rfah Bound to E. Coli Transcription Complex Paused at Ops Site (Not Complementary Scaffold)
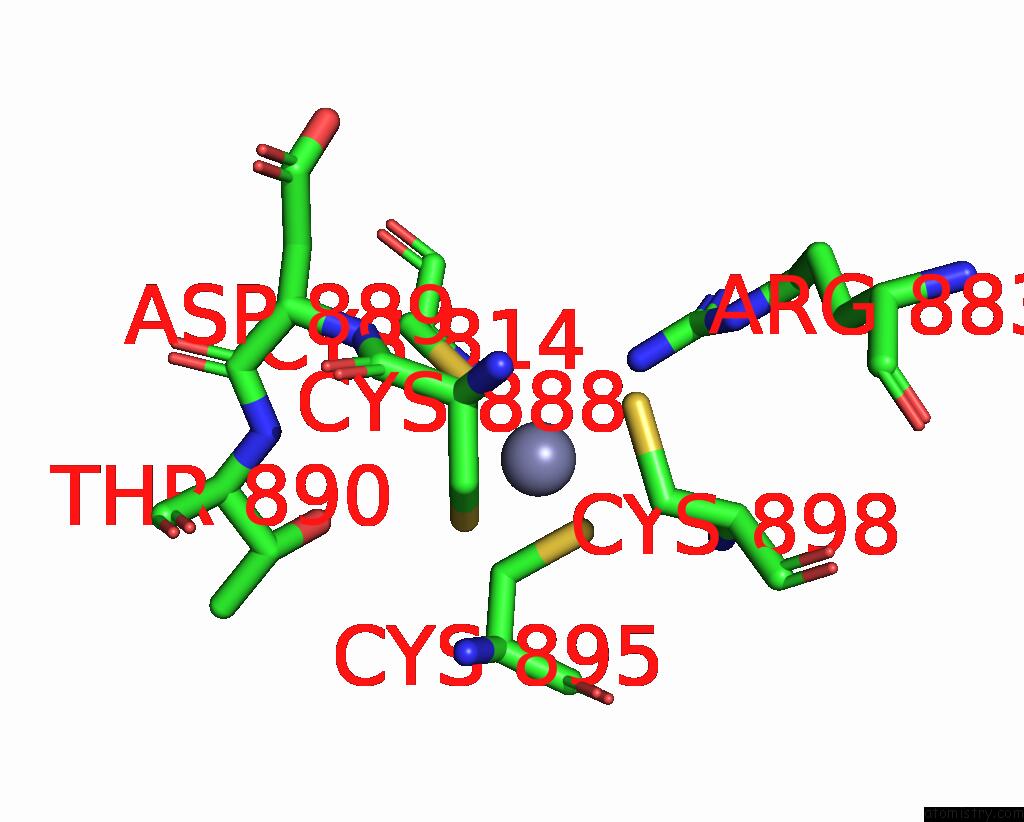
Mono view
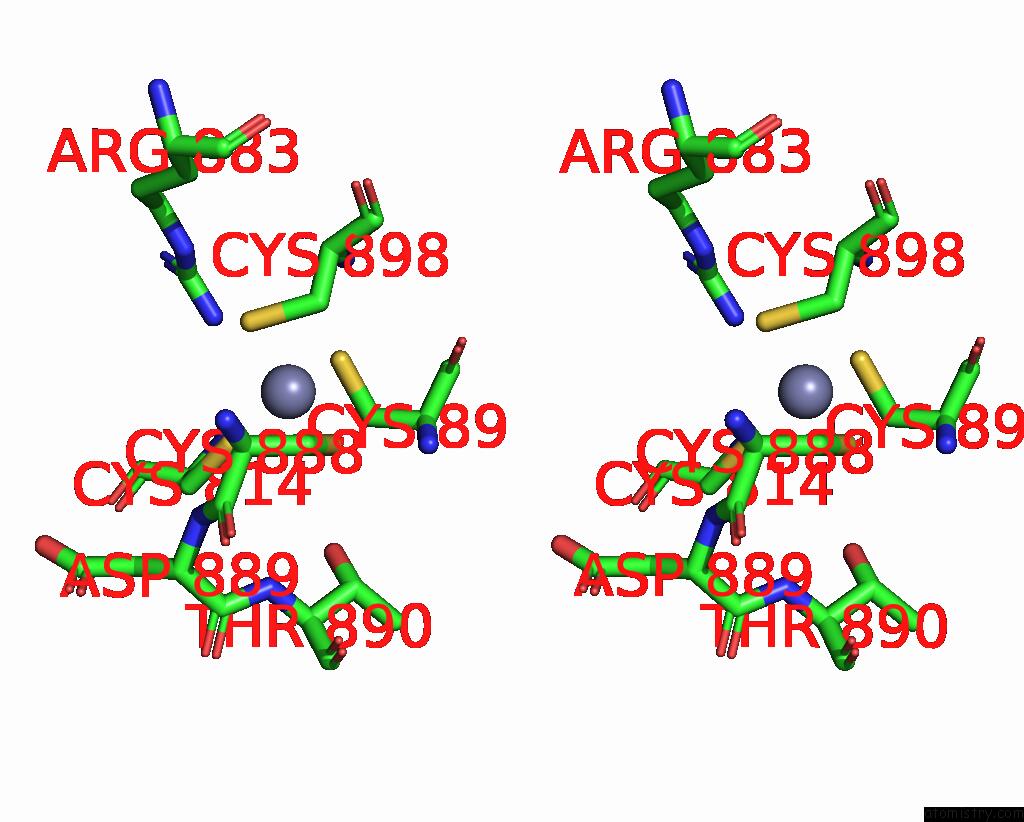
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of Fully Recruited Rfah Bound to E. Coli Transcription Complex Paused at Ops Site (Not Complementary Scaffold) within 5.0Å range:
|
Reference:
P.K.Zuber,
N.Said,
T.Hilal,
B.Wang,
B.Loll,
J.Gonzalez-Higueras,
C.A.Ramirez-Sarmiento,
G.A.Belogurov,
I.Artsimovitch,
M.C.Wahl,
S.H.Knauer.
Concerted Transformation of A Hyper-Paused Transcription Complex and Its Reinforcing Protein. Nat Commun V. 15 3040 2024.
ISSN: ESSN 2041-1723
PubMed: 38589445
DOI: 10.1038/S41467-024-47368-4
Page generated: Fri Aug 22 12:23:25 2025
ISSN: ESSN 2041-1723
PubMed: 38589445
DOI: 10.1038/S41467-024-47368-4
Last articles
Zn in 8ZGAZn in 8ZG9
Zn in 8ZIN
Zn in 8ZI8
Zn in 8ZHY
Zn in 8ZGB
Zn in 8ZCR
Zn in 8ZG8
Zn in 8ZEP
Zn in 8ZEO