Zinc »
PDB 7t85-7ts7 »
7tj4 »
Zinc in PDB 7tj4: Structure of the S. Aureus Amidase Lyth and Activator Acth Extracellular Domains
Enzymatic activity of Structure of the S. Aureus Amidase Lyth and Activator Acth Extracellular Domains
All present enzymatic activity of Structure of the S. Aureus Amidase Lyth and Activator Acth Extracellular Domains:
3.4.21.105;
3.4.21.105;
Protein crystallography data
The structure of Structure of the S. Aureus Amidase Lyth and Activator Acth Extracellular Domains, PDB code: 7tj4
was solved by
J.E.Page,
M.A.Skiba,
A.C.Kruse,
S.Walker,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 47.39 / 1.80 |
| Space group | P 2 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 44.6, 71.26, 190.4, 90, 90, 90 |
| R / Rfree (%) | 18.6 / 22.9 |
Zinc Binding Sites:
The binding sites of Zinc atom in the Structure of the S. Aureus Amidase Lyth and Activator Acth Extracellular Domains
(pdb code 7tj4). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 2 binding sites of Zinc where determined in the Structure of the S. Aureus Amidase Lyth and Activator Acth Extracellular Domains, PDB code: 7tj4:
Jump to Zinc binding site number: 1; 2;
In total 2 binding sites of Zinc where determined in the Structure of the S. Aureus Amidase Lyth and Activator Acth Extracellular Domains, PDB code: 7tj4:
Jump to Zinc binding site number: 1; 2;
Zinc binding site 1 out of 2 in 7tj4
Go back to
Zinc binding site 1 out
of 2 in the Structure of the S. Aureus Amidase Lyth and Activator Acth Extracellular Domains
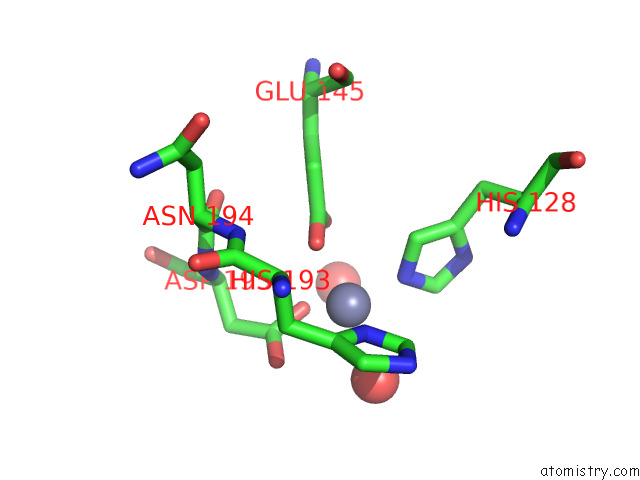
Mono view
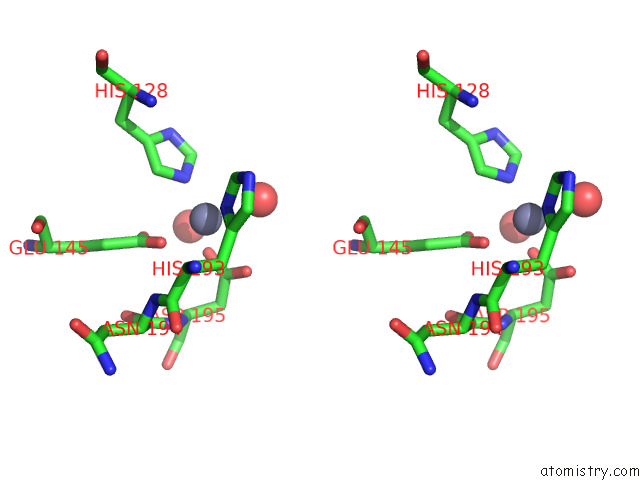
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Structure of the S. Aureus Amidase Lyth and Activator Acth Extracellular Domains within 5.0Å range:
|
Zinc binding site 2 out of 2 in 7tj4
Go back to
Zinc binding site 2 out
of 2 in the Structure of the S. Aureus Amidase Lyth and Activator Acth Extracellular Domains
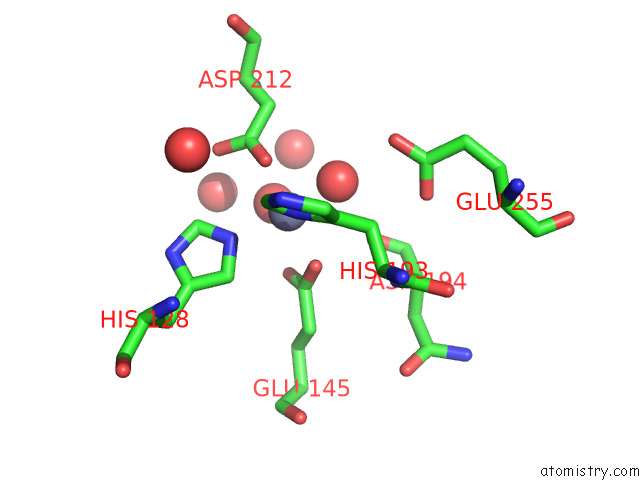
Mono view
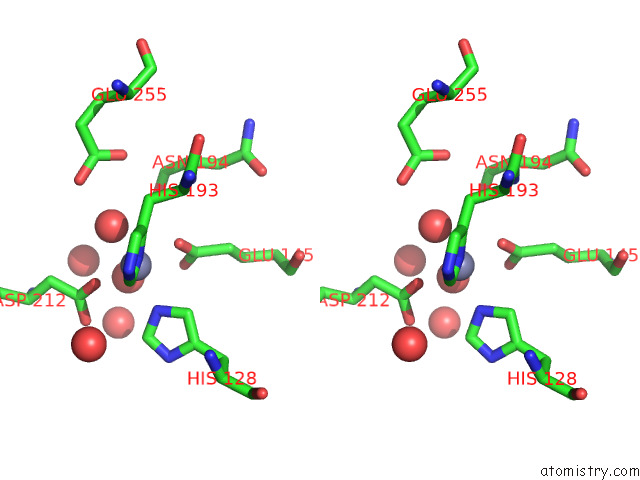
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of Structure of the S. Aureus Amidase Lyth and Activator Acth Extracellular Domains within 5.0Å range:
|
Reference:
J.E.Page,
M.A.Skiba,
T.Do,
A.C.Kruse,
S.Walker.
Metal Cofactor Stabilization By A Partner Protein Is A Widespread Strategy Employed For Amidase Activation. Proc.Natl.Acad.Sci.Usa V. 119 41119 2022.
ISSN: ESSN 1091-6490
PubMed: 35733252
DOI: 10.1073/PNAS.2201141119
Page generated: Fri Aug 22 04:58:48 2025
ISSN: ESSN 1091-6490
PubMed: 35733252
DOI: 10.1073/PNAS.2201141119
Last articles
Zn in 8DZFZn in 8DZE
Zn in 8DZJ
Zn in 8DY9
Zn in 8DY7
Zn in 8DYQ
Zn in 8DWL
Zn in 8DWJ
Zn in 8DQN
Zn in 8DW6