Zinc »
PDB 6kc5-6knb »
6km6 »
Zinc in PDB 6km6: Human Carbonic Anhydrase II Native 15 Atm CO2
Enzymatic activity of Human Carbonic Anhydrase II Native 15 Atm CO2
All present enzymatic activity of Human Carbonic Anhydrase II Native 15 Atm CO2:
4.2.1.1;
4.2.1.1;
Protein crystallography data
The structure of Human Carbonic Anhydrase II Native 15 Atm CO2, PDB code: 6km6
was solved by
C.U.Kim,
J.K.Kim,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 29.06 / 1.15 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 42.153, 41.308, 71.964, 90.00, 104.09, 90.00 |
| R / Rfree (%) | 10.5 / 13.4 |
Zinc Binding Sites:
The binding sites of Zinc atom in the Human Carbonic Anhydrase II Native 15 Atm CO2
(pdb code 6km6). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total only one binding site of Zinc was determined in the Human Carbonic Anhydrase II Native 15 Atm CO2, PDB code: 6km6:
In total only one binding site of Zinc was determined in the Human Carbonic Anhydrase II Native 15 Atm CO2, PDB code: 6km6:
Zinc binding site 1 out of 1 in 6km6
Go back to
Zinc binding site 1 out
of 1 in the Human Carbonic Anhydrase II Native 15 Atm CO2
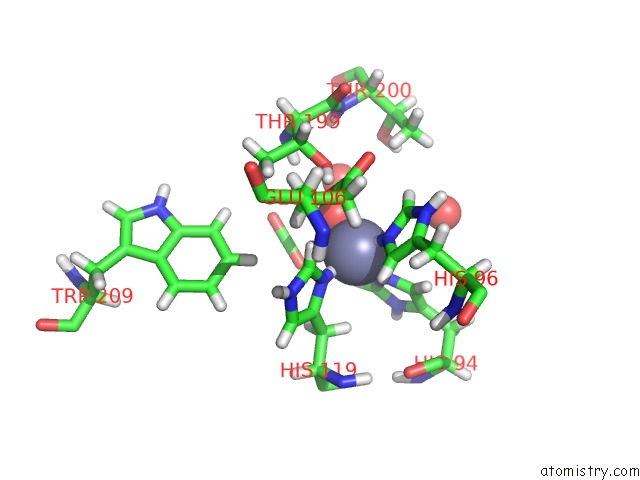
Mono view
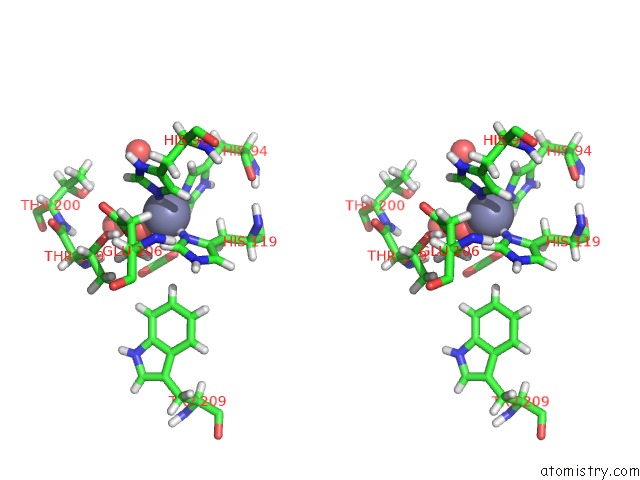
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Human Carbonic Anhydrase II Native 15 Atm CO2 within 5.0Å range:
|
Reference:
J.K.Kim,
C.Lee,
S.W.Lim,
J.T.Andring,
A.Adhikari,
R.Mckenna,
C.U.Kim.
Structural Insights Into the Effect of Active-Site Mutation on the Catalytic Mechanism of Carbonic Anhydrase. Iucrj V. 7 985 2020.
ISSN: ESSN 2052-2525
PubMed: 33209313
DOI: 10.1107/S2052252520011008
Page generated: Thu Aug 21 16:46:20 2025
ISSN: ESSN 2052-2525
PubMed: 33209313
DOI: 10.1107/S2052252520011008
Last articles
Zn in 6SEHZn in 6SEY
Zn in 6SEL
Zn in 6SE2
Zn in 6SDQ
Zn in 6SDM
Zn in 6SDT
Zn in 6SDS
Zn in 6SDN
Zn in 6SDP