Zinc »
PDB 6iv1-6j4g »
6iv2 »
Zinc in PDB 6iv2: Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae with A Mutation Y211A
Protein crystallography data
The structure of Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae with A Mutation Y211A, PDB code: 6iv2
was solved by
S.Chen,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 71.77 / 2.62 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 114.160, 160.109, 162.035, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 22.4 / 26.3 |
Zinc Binding Sites:
Pages:
>>> Page 1 <<< Page 2, Binding sites: 11 - 12;Binding sites:
The binding sites of Zinc atom in the Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae with A Mutation Y211A (pdb code 6iv2). This binding sites where shown within 5.0 Angstroms radius around Zinc atom.In total 12 binding sites of Zinc where determined in the Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae with A Mutation Y211A, PDB code: 6iv2:
Jump to Zinc binding site number: 1; 2; 3; 4; 5; 6; 7; 8; 9; 10;
Zinc binding site 1 out of 12 in 6iv2
Go back to
Zinc binding site 1 out
of 12 in the Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae with A Mutation Y211A
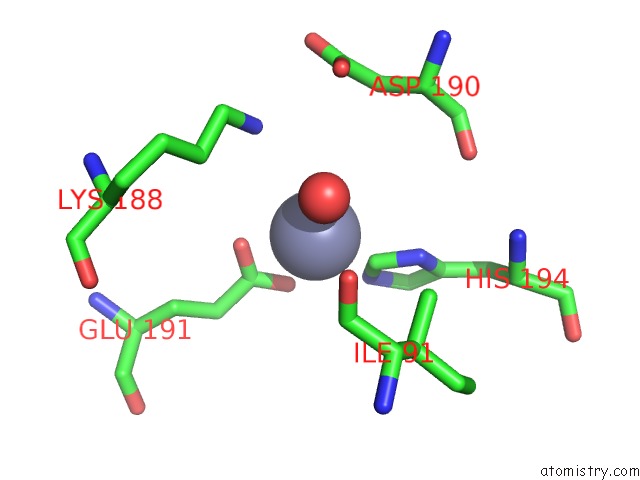
Mono view
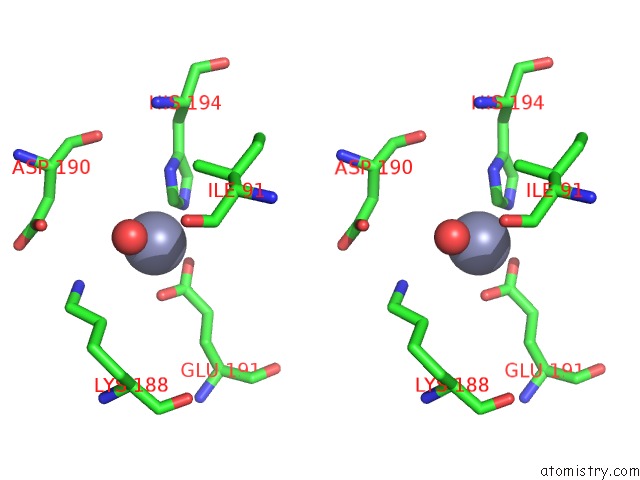
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae with A Mutation Y211A within 5.0Å range:
|
Zinc binding site 2 out of 12 in 6iv2
Go back to
Zinc binding site 2 out
of 12 in the Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae with A Mutation Y211A
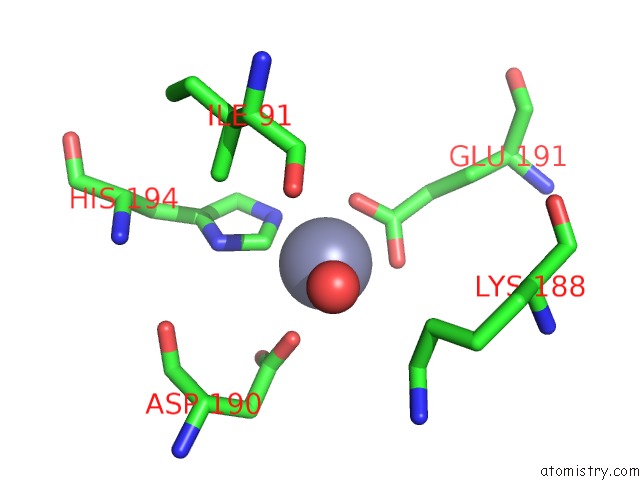
Mono view
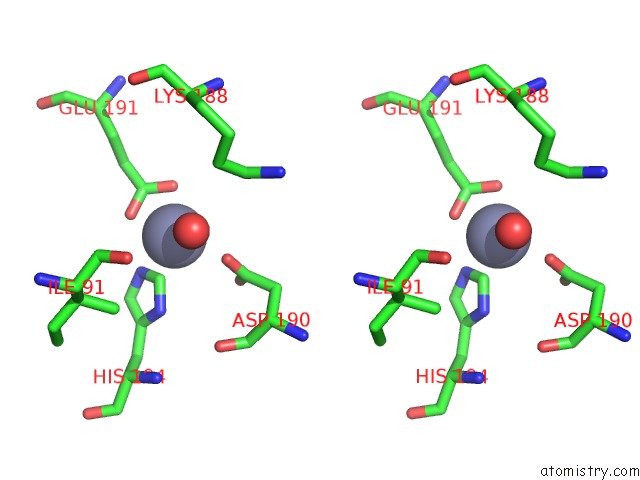
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae with A Mutation Y211A within 5.0Å range:
|
Zinc binding site 3 out of 12 in 6iv2
Go back to
Zinc binding site 3 out
of 12 in the Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae with A Mutation Y211A
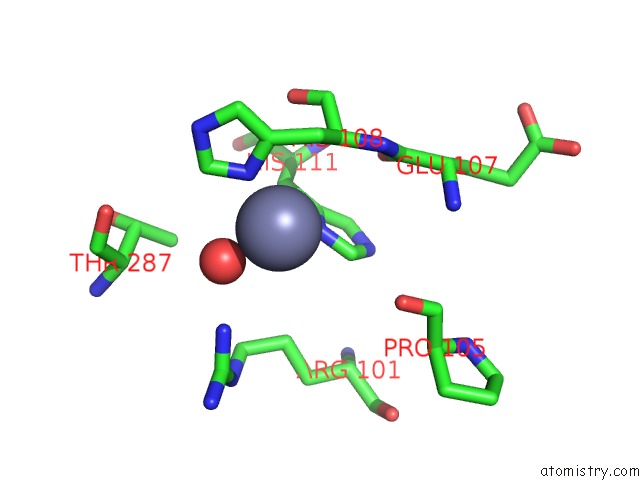
Mono view
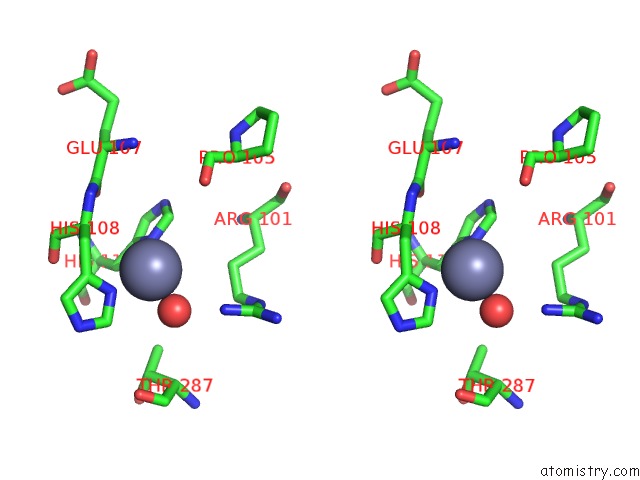
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 3 of Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae with A Mutation Y211A within 5.0Å range:
|
Zinc binding site 4 out of 12 in 6iv2
Go back to
Zinc binding site 4 out
of 12 in the Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae with A Mutation Y211A
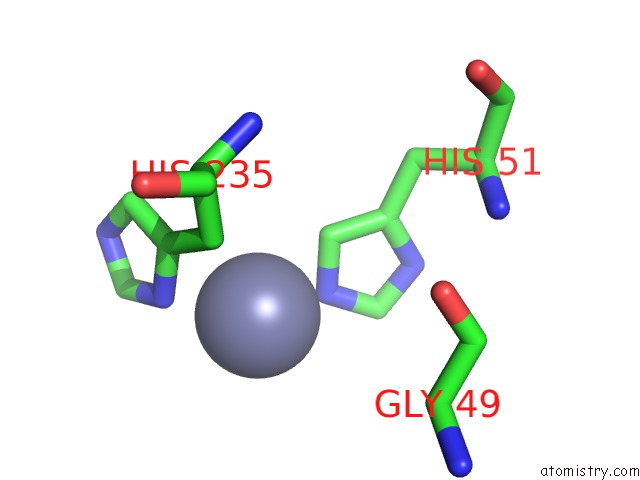
Mono view
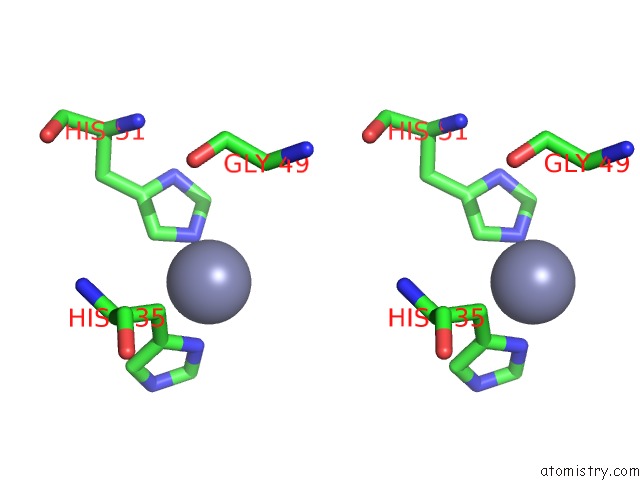
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 4 of Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae with A Mutation Y211A within 5.0Å range:
|
Zinc binding site 5 out of 12 in 6iv2
Go back to
Zinc binding site 5 out
of 12 in the Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae with A Mutation Y211A
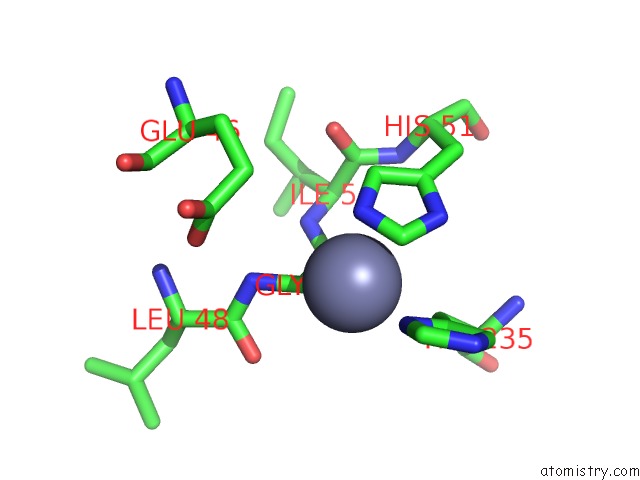
Mono view
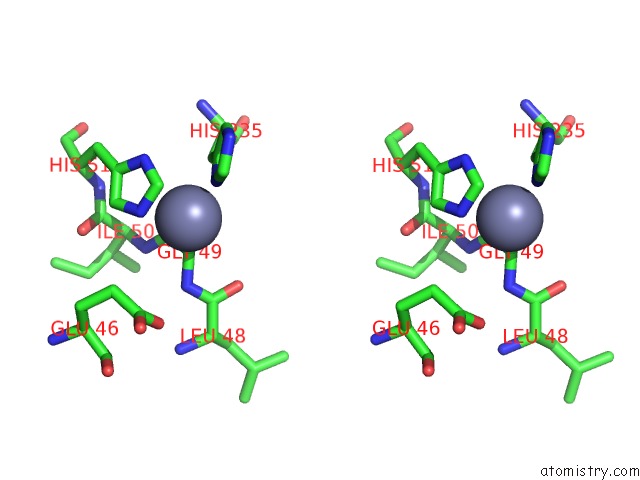
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 5 of Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae with A Mutation Y211A within 5.0Å range:
|
Zinc binding site 6 out of 12 in 6iv2
Go back to
Zinc binding site 6 out
of 12 in the Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae with A Mutation Y211A
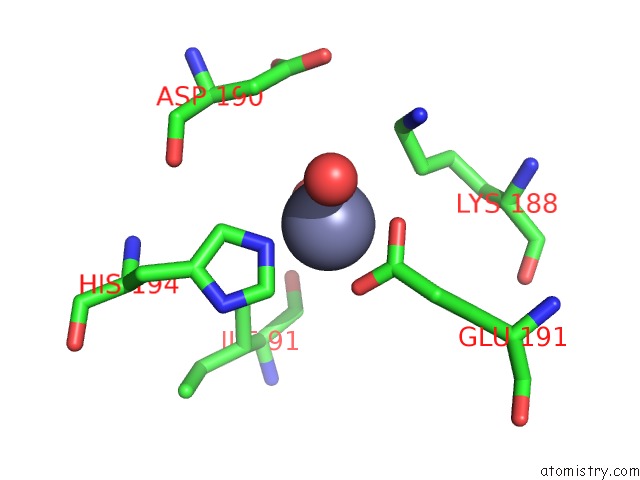
Mono view
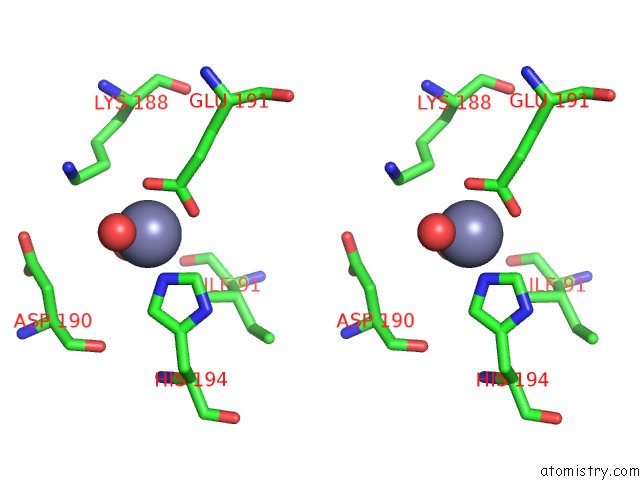
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 6 of Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae with A Mutation Y211A within 5.0Å range:
|
Zinc binding site 7 out of 12 in 6iv2
Go back to
Zinc binding site 7 out
of 12 in the Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae with A Mutation Y211A
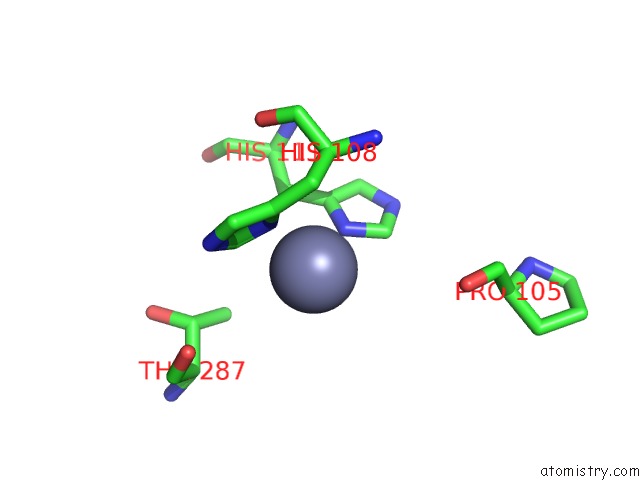
Mono view
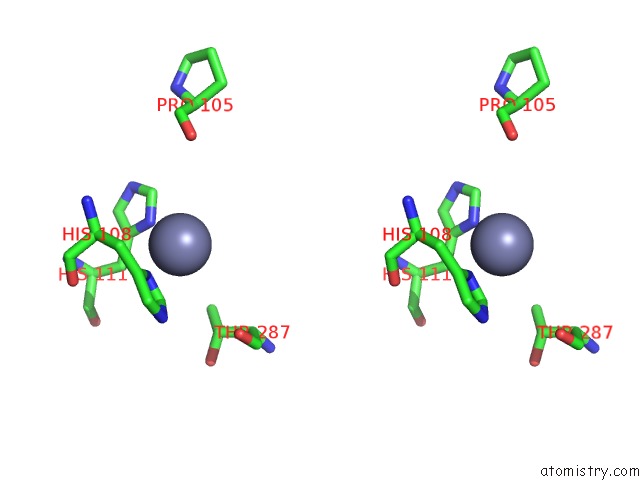
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 7 of Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae with A Mutation Y211A within 5.0Å range:
|
Zinc binding site 8 out of 12 in 6iv2
Go back to
Zinc binding site 8 out
of 12 in the Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae with A Mutation Y211A
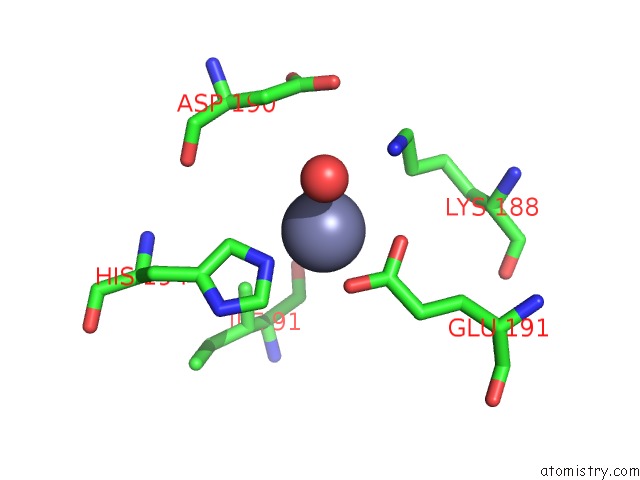
Mono view
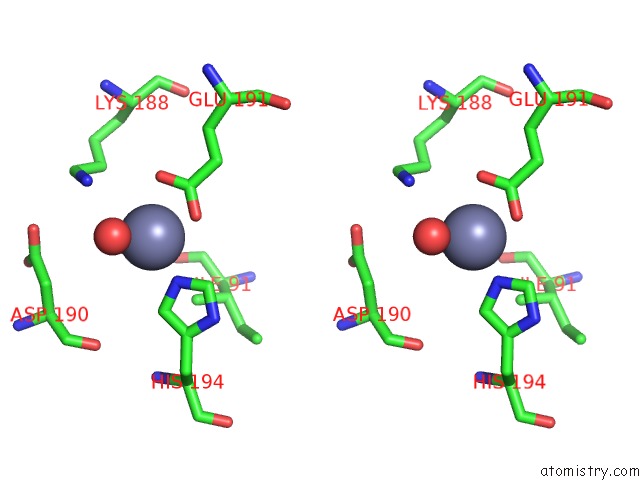
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 8 of Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae with A Mutation Y211A within 5.0Å range:
|
Zinc binding site 9 out of 12 in 6iv2
Go back to
Zinc binding site 9 out
of 12 in the Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae with A Mutation Y211A
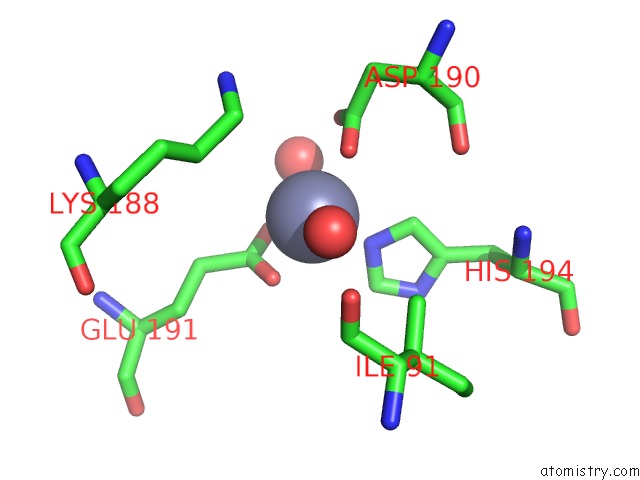
Mono view
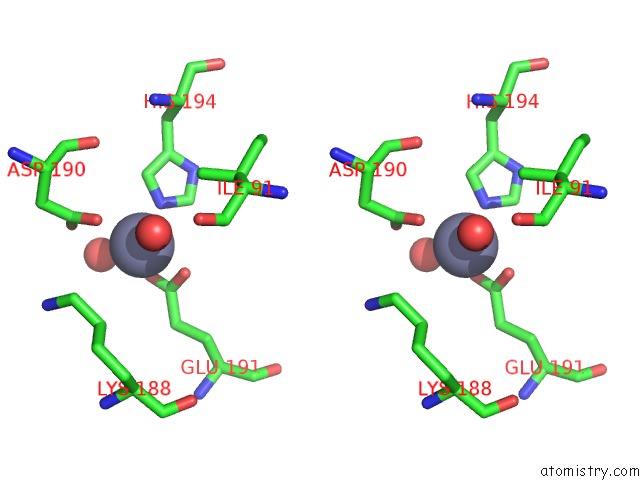
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 9 of Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae with A Mutation Y211A within 5.0Å range:
|
Zinc binding site 10 out of 12 in 6iv2
Go back to
Zinc binding site 10 out
of 12 in the Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae with A Mutation Y211A
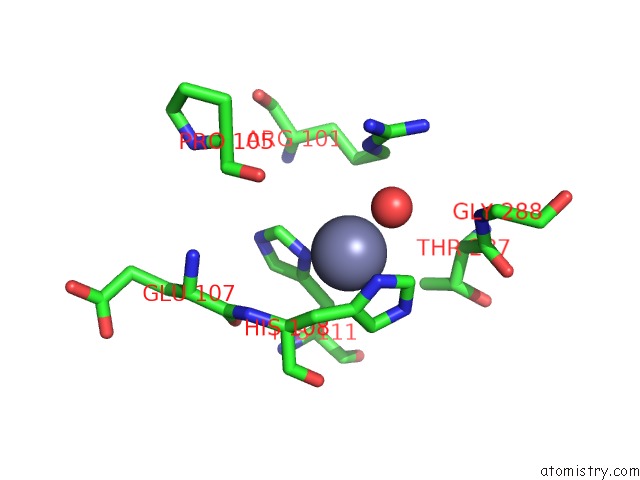
Mono view
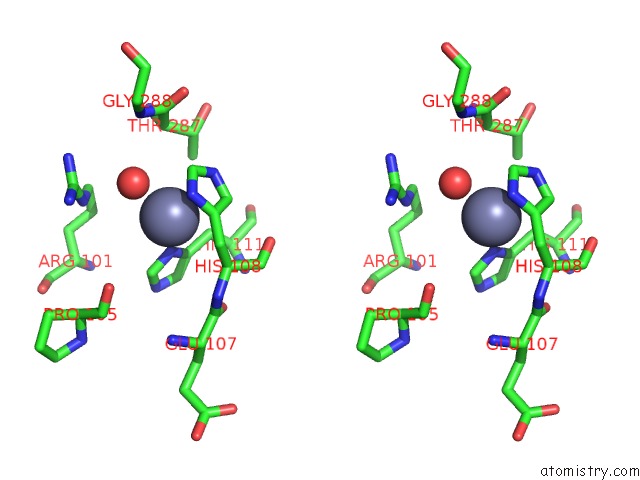
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 10 of Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae with A Mutation Y211A within 5.0Å range:
|
Reference:
C.Ji,
A.Kittredge,
A.Hopiavuori,
N.Ward,
S.Chen,
Y.Fukuda,
Y.Zhang,
T.Yang.
Dual CA2+-Dependent Gates in Human BESTROPHIN1 Underlie Disease-Causing Mechanisms of Gain-of-Function Mutations. Commun Biol V. 2 240 2019.
ISSN: ESSN 2399-3642
PubMed: 31263784
DOI: 10.1038/S42003-019-0433-3
Page generated: Thu Aug 21 15:57:26 2025
ISSN: ESSN 2399-3642
PubMed: 31263784
DOI: 10.1038/S42003-019-0433-3
Last articles
Zn in 6RD8Zn in 6RCW
Zn in 6RD7
Zn in 6RD5
Zn in 6RD4
Zn in 6RD0
Zn in 6RCN
Zn in 6RCL
Zn in 6RBI
Zn in 6RBC