Zinc »
PDB 5kb0-5kj1 »
5kgl »
Zinc in PDB 5kgl: 2.45A Resolution Structure of Apo Independent Phosphoglycerate Mutase From C. Elegans (Orthorhombic Form)
Enzymatic activity of 2.45A Resolution Structure of Apo Independent Phosphoglycerate Mutase From C. Elegans (Orthorhombic Form)
All present enzymatic activity of 2.45A Resolution Structure of Apo Independent Phosphoglycerate Mutase From C. Elegans (Orthorhombic Form):
5.4.2.12;
5.4.2.12;
Protein crystallography data
The structure of 2.45A Resolution Structure of Apo Independent Phosphoglycerate Mutase From C. Elegans (Orthorhombic Form), PDB code: 5kgl
was solved by
S.Lovell,
N.Mehzabeen,
K.P.Battaile,
H.Yu,
P.Dranchak,
R.Macarthur,
Z.Li,
T.Carlow,
H.Suga,
J.Inglese,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 40.44 / 2.45 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 70.321, 98.852, 173.144, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 18.5 / 26 |
Other elements in 5kgl:
The structure of 2.45A Resolution Structure of Apo Independent Phosphoglycerate Mutase From C. Elegans (Orthorhombic Form) also contains other interesting chemical elements:
| Manganese | (Mn) | 2 atoms |
| Chlorine | (Cl) | 2 atoms |
Zinc Binding Sites:
The binding sites of Zinc atom in the 2.45A Resolution Structure of Apo Independent Phosphoglycerate Mutase From C. Elegans (Orthorhombic Form)
(pdb code 5kgl). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 2 binding sites of Zinc where determined in the 2.45A Resolution Structure of Apo Independent Phosphoglycerate Mutase From C. Elegans (Orthorhombic Form), PDB code: 5kgl:
Jump to Zinc binding site number: 1; 2;
In total 2 binding sites of Zinc where determined in the 2.45A Resolution Structure of Apo Independent Phosphoglycerate Mutase From C. Elegans (Orthorhombic Form), PDB code: 5kgl:
Jump to Zinc binding site number: 1; 2;
Zinc binding site 1 out of 2 in 5kgl
Go back to
Zinc binding site 1 out
of 2 in the 2.45A Resolution Structure of Apo Independent Phosphoglycerate Mutase From C. Elegans (Orthorhombic Form)
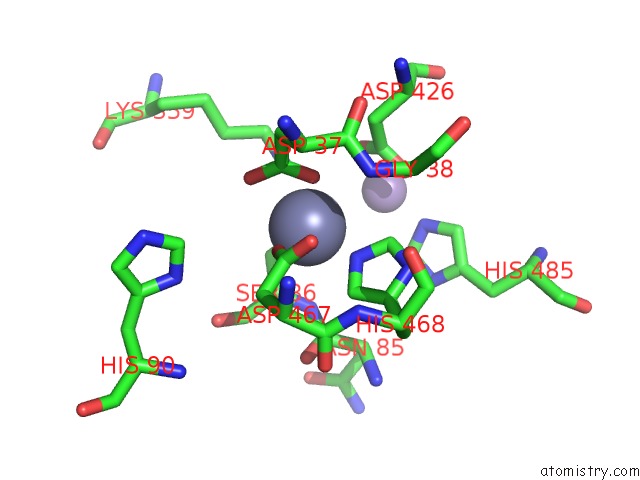
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of 2.45A Resolution Structure of Apo Independent Phosphoglycerate Mutase From C. Elegans (Orthorhombic Form) within 5.0Å range:
|
Zinc binding site 2 out of 2 in 5kgl
Go back to
Zinc binding site 2 out
of 2 in the 2.45A Resolution Structure of Apo Independent Phosphoglycerate Mutase From C. Elegans (Orthorhombic Form)

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of 2.45A Resolution Structure of Apo Independent Phosphoglycerate Mutase From C. Elegans (Orthorhombic Form) within 5.0Å range:
|
Reference:
H.Yu,
P.Dranchak,
Z.Li,
R.Macarthur,
M.S.Munson,
N.Mehzabeen,
N.J.Baird,
K.P.Battalie,
D.Ross,
S.Lovell,
C.K.Carlow,
H.Suga,
J.Inglese.
Macrocycle Peptides Delineate Locked-Open Inhibition Mechanism For Microorganism Phosphoglycerate Mutases. Nat Commun V. 8 14932 2017.
ISSN: ESSN 2041-1723
PubMed: 28368002
DOI: 10.1038/NCOMMS14932
Page generated: Sun Oct 27 20:23:54 2024
ISSN: ESSN 2041-1723
PubMed: 28368002
DOI: 10.1038/NCOMMS14932
Last articles
Al in 3C7KAl in 3B9R
Al in 3AB3
Al in 3BH7
Al in 3AR8
Al in 2YNM
Al in 2ZJY
Al in 2ZBG
Al in 2ZBD
Al in 2Y3I