Zinc »
PDB 4w9y-4wnv »
4wd8 »
Zinc in PDB 4wd8: Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae
Protein crystallography data
The structure of Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae, PDB code: 4wd8
was solved by
T.Yang,
Q.Liu,
W.A.Hendrickson,
New York Consortium On Membrane Proteinstructure (Nycomps),
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 38.96 / 2.30 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 114.079, 160.034, 161.881, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 19.6 / 21.5 |
Zinc Binding Sites:
Pages:
>>> Page 1 <<< Page 2, Binding sites: 11 - 15;Binding sites:
The binding sites of Zinc atom in the Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae (pdb code 4wd8). This binding sites where shown within 5.0 Angstroms radius around Zinc atom.In total 15 binding sites of Zinc where determined in the Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae, PDB code: 4wd8:
Jump to Zinc binding site number: 1; 2; 3; 4; 5; 6; 7; 8; 9; 10;
Zinc binding site 1 out of 15 in 4wd8
Go back to
Zinc binding site 1 out
of 15 in the Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae within 5.0Å range:
|
Zinc binding site 2 out of 15 in 4wd8
Go back to
Zinc binding site 2 out
of 15 in the Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae within 5.0Å range:
|
Zinc binding site 3 out of 15 in 4wd8
Go back to
Zinc binding site 3 out
of 15 in the Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 3 of Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae within 5.0Å range:
|
Zinc binding site 4 out of 15 in 4wd8
Go back to
Zinc binding site 4 out
of 15 in the Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 4 of Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae within 5.0Å range:
|
Zinc binding site 5 out of 15 in 4wd8
Go back to
Zinc binding site 5 out
of 15 in the Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 5 of Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae within 5.0Å range:
|
Zinc binding site 6 out of 15 in 4wd8
Go back to
Zinc binding site 6 out
of 15 in the Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae
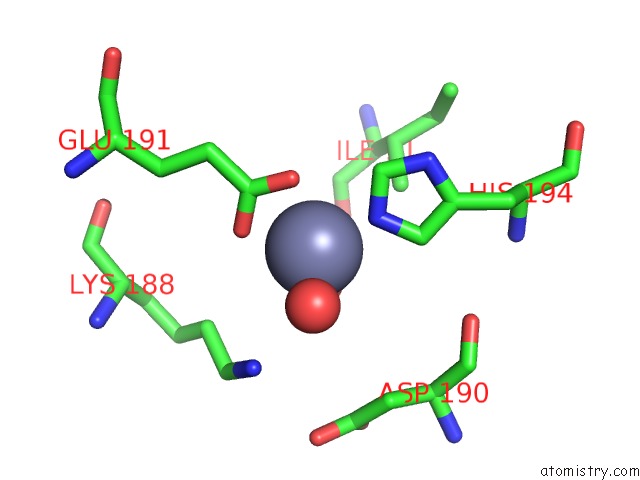
Mono view
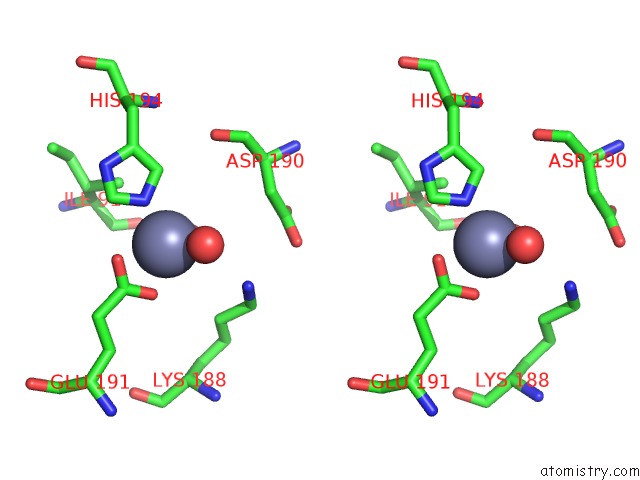
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 6 of Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae within 5.0Å range:
|
Zinc binding site 7 out of 15 in 4wd8
Go back to
Zinc binding site 7 out
of 15 in the Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae

Mono view
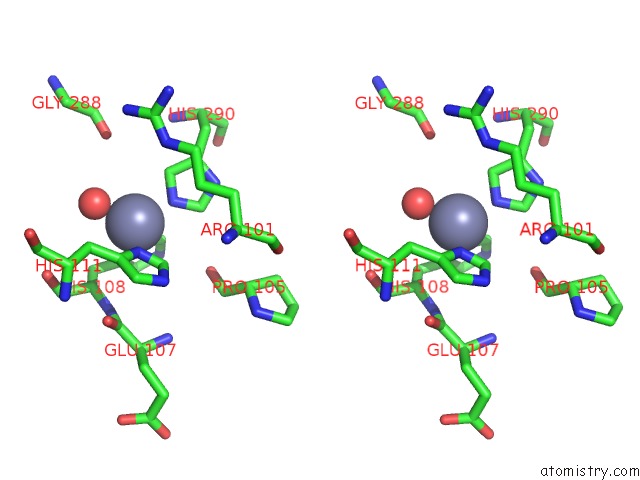
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 7 of Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae within 5.0Å range:
|
Zinc binding site 8 out of 15 in 4wd8
Go back to
Zinc binding site 8 out
of 15 in the Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 8 of Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae within 5.0Å range:
|
Zinc binding site 9 out of 15 in 4wd8
Go back to
Zinc binding site 9 out
of 15 in the Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 9 of Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae within 5.0Å range:
|
Zinc binding site 10 out of 15 in 4wd8
Go back to
Zinc binding site 10 out
of 15 in the Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 10 of Crystal Structure of A Bacterial Bestrophin Homolog From Klebsiella Pneumoniae within 5.0Å range:
|
Reference:
T.Yang,
Q.Liu,
B.Kloss,
R.Bruni,
R.C.Kalathur,
Y.Guo,
E.Kloppmann,
B.Rost,
H.M.Colecraft,
W.A.Hendrickson.
Structure and Selectivity in Bestrophin Ion Channels. Science V. 346 355 2014.
ISSN: ESSN 1095-9203
PubMed: 25324390
DOI: 10.1126/SCIENCE.1259723
Page generated: Wed Aug 20 23:04:11 2025
ISSN: ESSN 1095-9203
PubMed: 25324390
DOI: 10.1126/SCIENCE.1259723
Last articles
Zn in 5M64Zn in 5M6N
Zn in 5M78
Zn in 5M6M
Zn in 5M6L
Zn in 5M6H
Zn in 5M6F
Zn in 5M69
Zn in 5M6E
Zn in 5M5Y