Zinc »
PDB 4r76-4rm5 »
4rm5 »
Zinc in PDB 4rm5: Structural and Mechanistic Insights Into Ndm-1 Catalyzed Hydrolysis of Cephalosporins
Enzymatic activity of Structural and Mechanistic Insights Into Ndm-1 Catalyzed Hydrolysis of Cephalosporins
All present enzymatic activity of Structural and Mechanistic Insights Into Ndm-1 Catalyzed Hydrolysis of Cephalosporins:
3.5.2.6;
3.5.2.6;
Protein crystallography data
The structure of Structural and Mechanistic Insights Into Ndm-1 Catalyzed Hydrolysis of Cephalosporins, PDB code: 4rm5
was solved by
H.Feng,
J.Ding,
D.Zhu,
X.Liu,
X.Xu,
Y.Zhang,
S.Zang,
D.-C.Wang,
W.Liu,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 35.02 / 2.10 |
| Space group | P 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 46.100, 68.120, 68.910, 87.62, 88.34, 77.42 |
| R / Rfree (%) | 15.9 / 19.2 |
Zinc Binding Sites:
The binding sites of Zinc atom in the Structural and Mechanistic Insights Into Ndm-1 Catalyzed Hydrolysis of Cephalosporins
(pdb code 4rm5). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 8 binding sites of Zinc where determined in the Structural and Mechanistic Insights Into Ndm-1 Catalyzed Hydrolysis of Cephalosporins, PDB code: 4rm5:
Jump to Zinc binding site number: 1; 2; 3; 4; 5; 6; 7; 8;
In total 8 binding sites of Zinc where determined in the Structural and Mechanistic Insights Into Ndm-1 Catalyzed Hydrolysis of Cephalosporins, PDB code: 4rm5:
Jump to Zinc binding site number: 1; 2; 3; 4; 5; 6; 7; 8;
Zinc binding site 1 out of 8 in 4rm5
Go back to
Zinc binding site 1 out
of 8 in the Structural and Mechanistic Insights Into Ndm-1 Catalyzed Hydrolysis of Cephalosporins
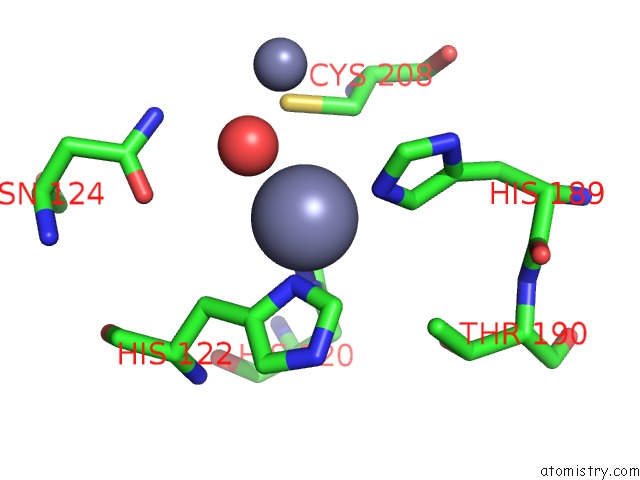
Mono view
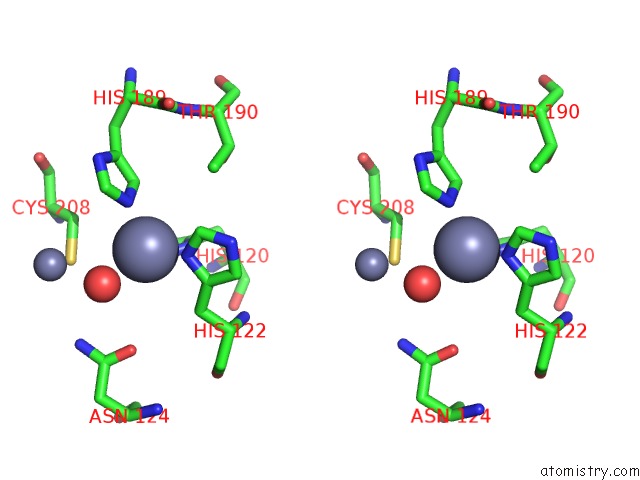
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Structural and Mechanistic Insights Into Ndm-1 Catalyzed Hydrolysis of Cephalosporins within 5.0Å range:
|
Zinc binding site 2 out of 8 in 4rm5
Go back to
Zinc binding site 2 out
of 8 in the Structural and Mechanistic Insights Into Ndm-1 Catalyzed Hydrolysis of Cephalosporins
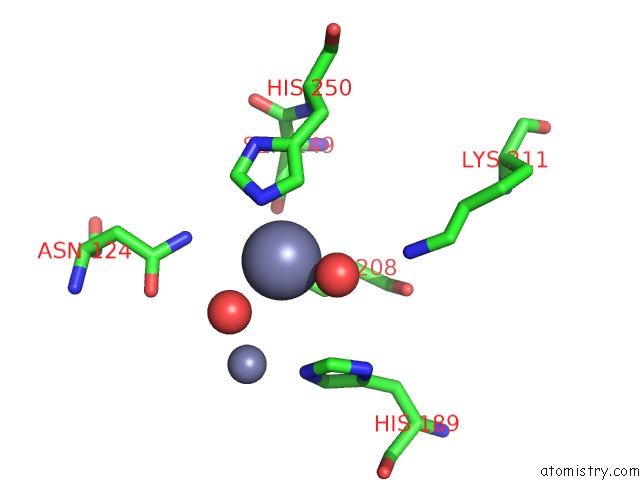
Mono view
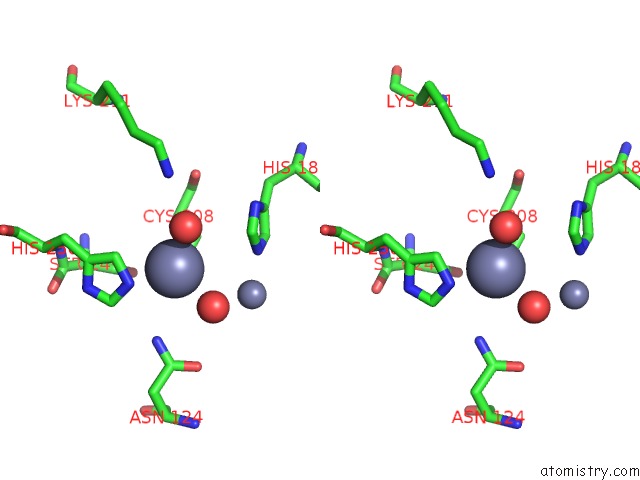
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of Structural and Mechanistic Insights Into Ndm-1 Catalyzed Hydrolysis of Cephalosporins within 5.0Å range:
|
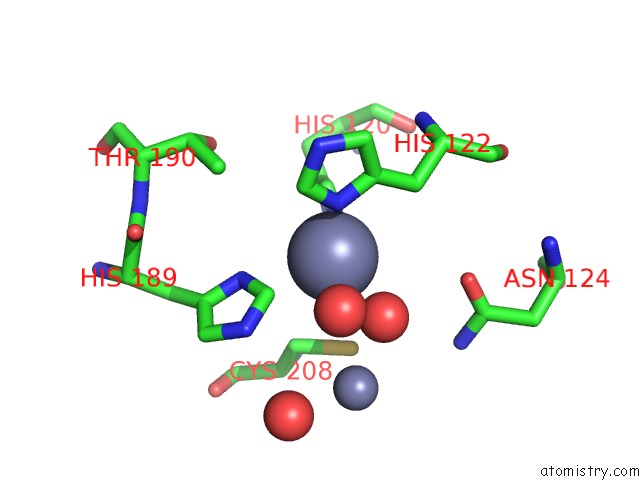
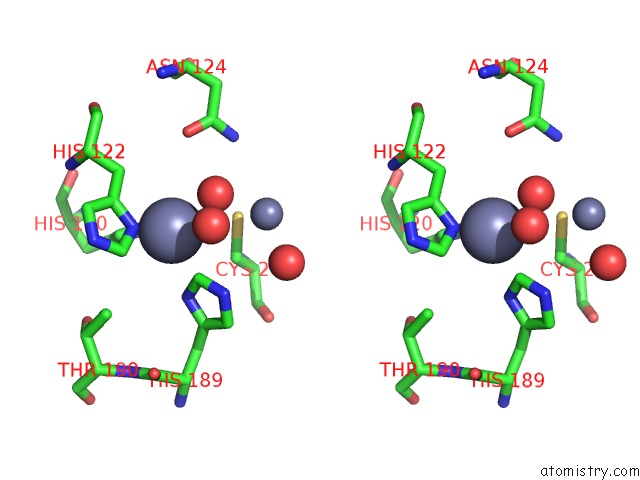
Zinc binding site 3 out of 8 in 4rm5
Go back to
Zinc binding site 3 out
of 8 in the Structural and Mechanistic Insights Into Ndm-1 Catalyzed Hydrolysis of Cephalosporins
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 3 of Structural and Mechanistic Insights Into Ndm-1 Catalyzed Hydrolysis of Cephalosporins within 5.0Å range:
|
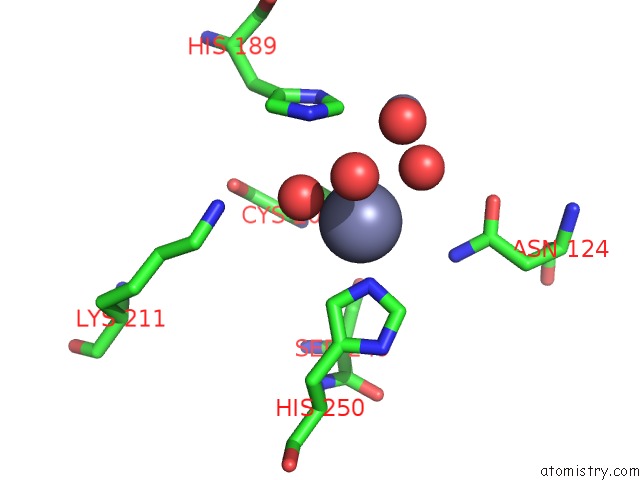
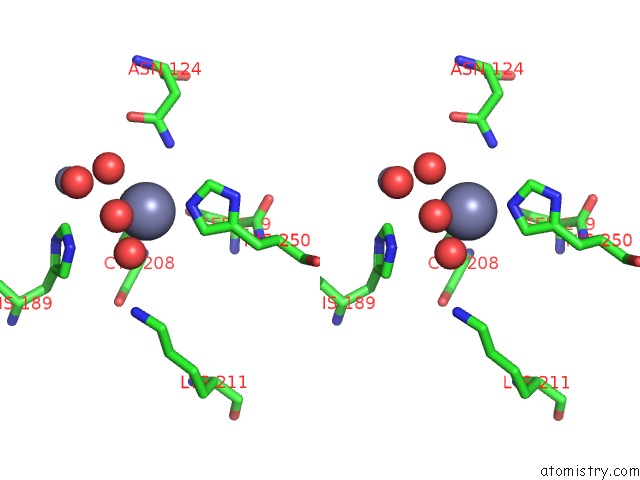
Zinc binding site 4 out of 8 in 4rm5
Go back to
Zinc binding site 4 out
of 8 in the Structural and Mechanistic Insights Into Ndm-1 Catalyzed Hydrolysis of Cephalosporins
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 4 of Structural and Mechanistic Insights Into Ndm-1 Catalyzed Hydrolysis of Cephalosporins within 5.0Å range:
|
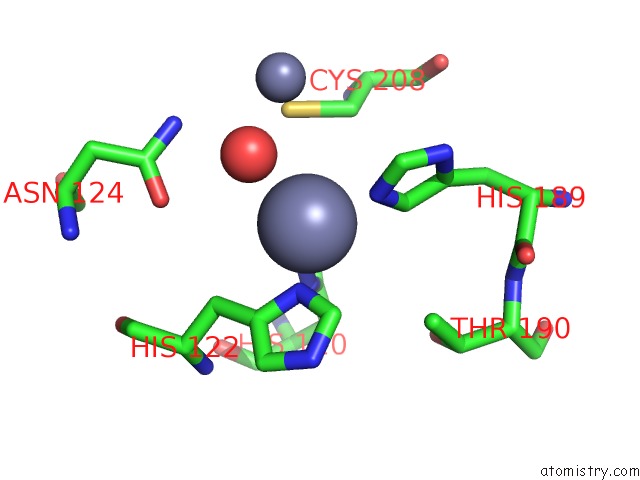
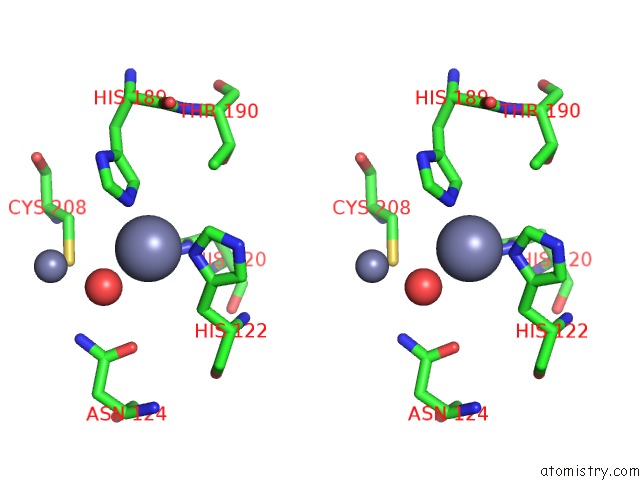
Zinc binding site 5 out of 8 in 4rm5
Go back to
Zinc binding site 5 out
of 8 in the Structural and Mechanistic Insights Into Ndm-1 Catalyzed Hydrolysis of Cephalosporins
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 5 of Structural and Mechanistic Insights Into Ndm-1 Catalyzed Hydrolysis of Cephalosporins within 5.0Å range:
|
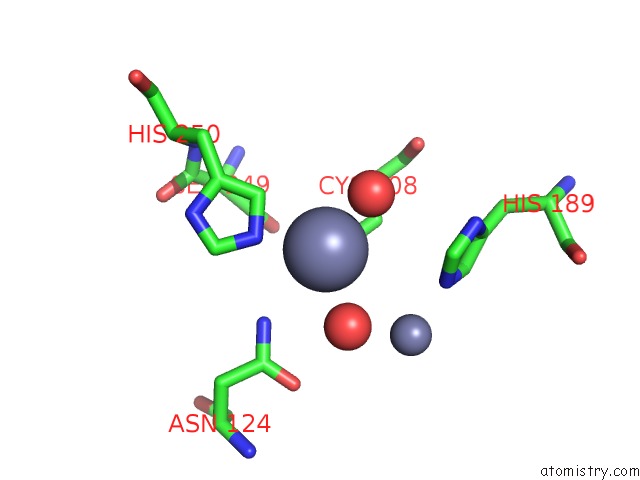
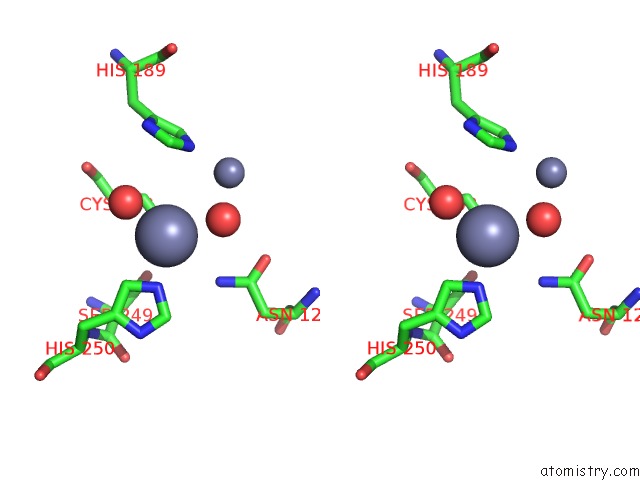
Zinc binding site 6 out of 8 in 4rm5
Go back to
Zinc binding site 6 out
of 8 in the Structural and Mechanistic Insights Into Ndm-1 Catalyzed Hydrolysis of Cephalosporins
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 6 of Structural and Mechanistic Insights Into Ndm-1 Catalyzed Hydrolysis of Cephalosporins within 5.0Å range:
|
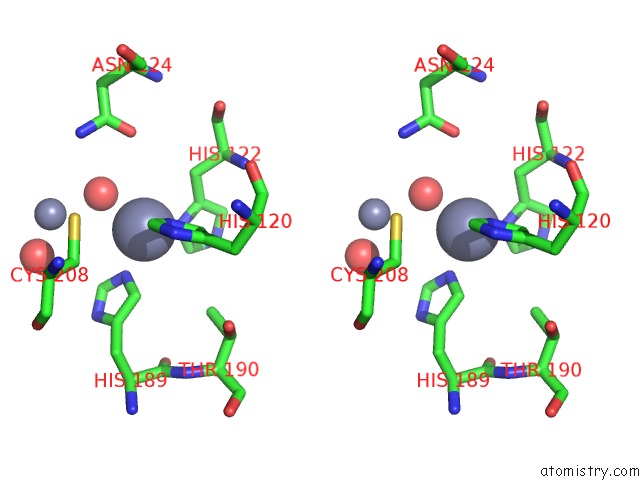
Zinc binding site 7 out of 8 in 4rm5
Go back to
Zinc binding site 7 out
of 8 in the Structural and Mechanistic Insights Into Ndm-1 Catalyzed Hydrolysis of Cephalosporins
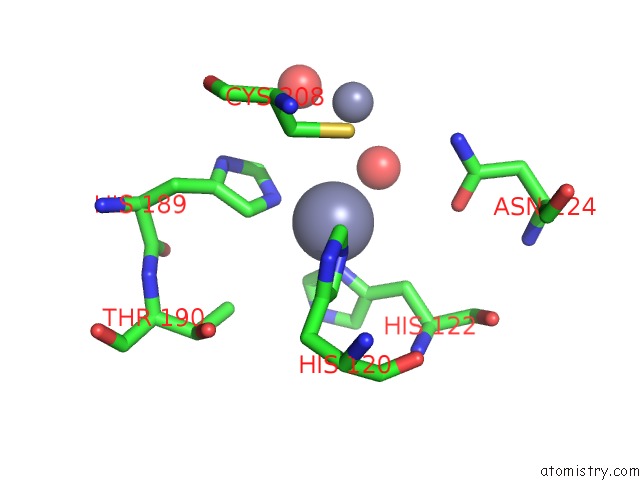
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 7 of Structural and Mechanistic Insights Into Ndm-1 Catalyzed Hydrolysis of Cephalosporins within 5.0Å range:
|
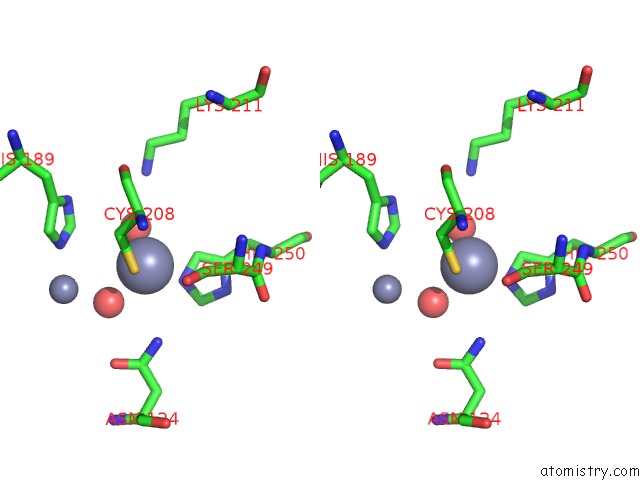
Zinc binding site 8 out of 8 in 4rm5
Go back to
Zinc binding site 8 out
of 8 in the Structural and Mechanistic Insights Into Ndm-1 Catalyzed Hydrolysis of Cephalosporins
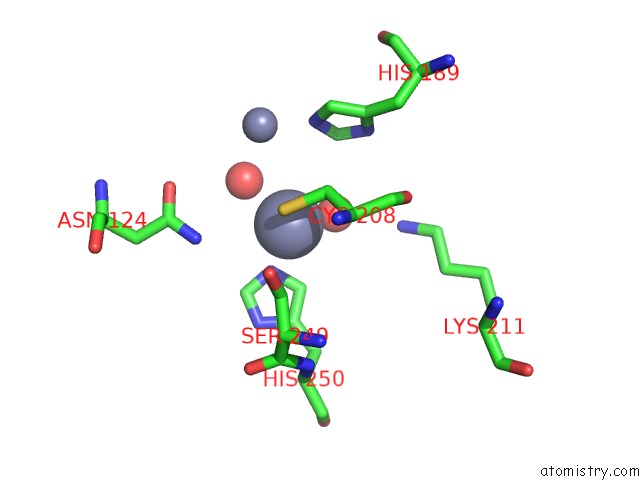
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 8 of Structural and Mechanistic Insights Into Ndm-1 Catalyzed Hydrolysis of Cephalosporins within 5.0Å range:
|
Reference:
H.Feng,
J.Ding,
D.Zhu,
X.Liu,
X.Xu,
Y.Zhang,
S.Zang,
D.C.Wang,
W.Liu.
Structural and Mechanistic Insights Into Ndm-1 Catalyzed Hydrolysis of Cephalosporins. J.Am.Chem.Soc. V. 136 14694 2014.
ISSN: ISSN 0002-7863
PubMed: 25268575
DOI: 10.1021/JA508388E
Page generated: Wed Aug 20 22:11:13 2025
ISSN: ISSN 0002-7863
PubMed: 25268575
DOI: 10.1021/JA508388E
Last articles
Zn in 5IY6Zn in 5IY7
Zn in 5IY0
Zn in 5IY5
Zn in 5IX2
Zn in 5IX1
Zn in 5IX0
Zn in 5IWG
Zn in 5IWA
Zn in 5IT5