Zinc »
PDB 4ad9-4arf »
4agq »
Zinc in PDB 4agq: Structure of the P53 Core Domain Mutant Y220C Bound to the Stabilizing Small Molecule PHIKAN5196
Protein crystallography data
The structure of Structure of the P53 Core Domain Mutant Y220C Bound to the Stabilizing Small Molecule PHIKAN5196, PDB code: 4agq
was solved by
A.C.Joerger,
R.Wilcken,
F.M.Boeckler,
A.R.Fersht,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 24.62 / 1.42 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 65.038, 71.242, 104.938, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 17.7 / 19.2 |
Other elements in 4agq:
The structure of Structure of the P53 Core Domain Mutant Y220C Bound to the Stabilizing Small Molecule PHIKAN5196 also contains other interesting chemical elements:
| Iodine | (I) | 2 atoms |
Zinc Binding Sites:
The binding sites of Zinc atom in the Structure of the P53 Core Domain Mutant Y220C Bound to the Stabilizing Small Molecule PHIKAN5196
(pdb code 4agq). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 2 binding sites of Zinc where determined in the Structure of the P53 Core Domain Mutant Y220C Bound to the Stabilizing Small Molecule PHIKAN5196, PDB code: 4agq:
Jump to Zinc binding site number: 1; 2;
In total 2 binding sites of Zinc where determined in the Structure of the P53 Core Domain Mutant Y220C Bound to the Stabilizing Small Molecule PHIKAN5196, PDB code: 4agq:
Jump to Zinc binding site number: 1; 2;
Zinc binding site 1 out of 2 in 4agq
Go back to
Zinc binding site 1 out
of 2 in the Structure of the P53 Core Domain Mutant Y220C Bound to the Stabilizing Small Molecule PHIKAN5196

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Structure of the P53 Core Domain Mutant Y220C Bound to the Stabilizing Small Molecule PHIKAN5196 within 5.0Å range:
|
Zinc binding site 2 out of 2 in 4agq
Go back to
Zinc binding site 2 out
of 2 in the Structure of the P53 Core Domain Mutant Y220C Bound to the Stabilizing Small Molecule PHIKAN5196
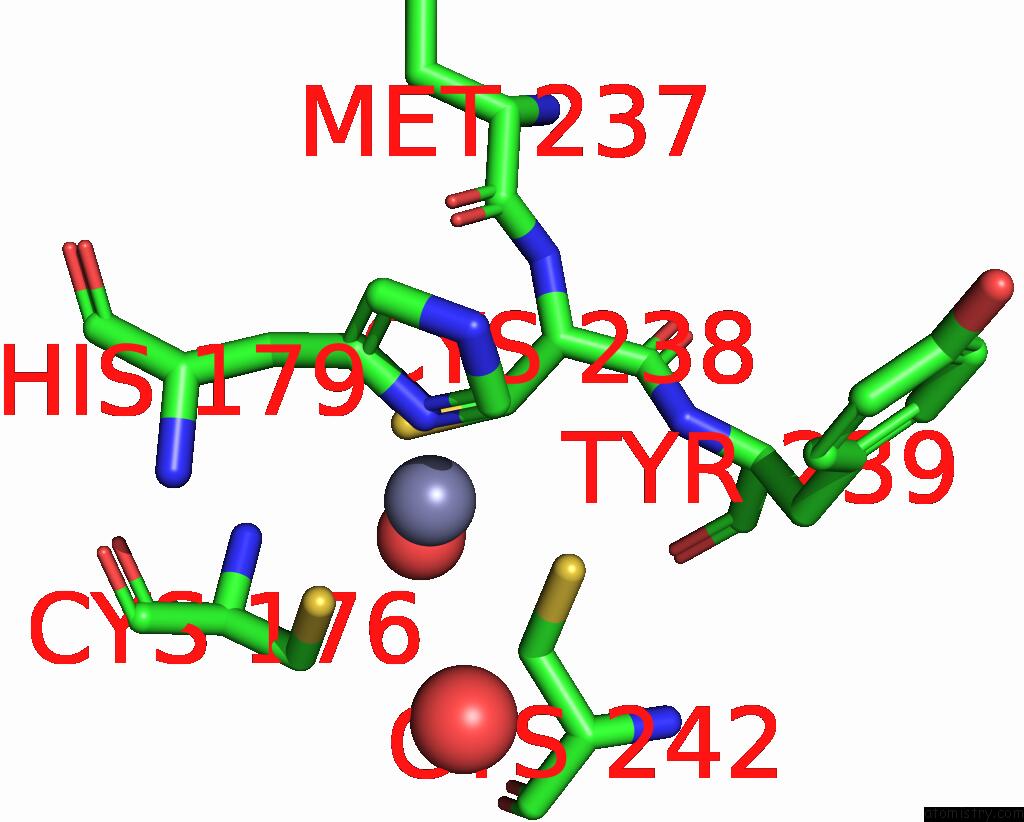
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of Structure of the P53 Core Domain Mutant Y220C Bound to the Stabilizing Small Molecule PHIKAN5196 within 5.0Å range:
|
Reference:
R.Wilcken,
X.Liu,
M.O.Zimmermann,
T.J.Rutherford,
A.R.Fersht,
A.C.Joerger,
F.M.Boeckler.
Halogen-Enriched Fragment Libraries As Leads For Drug Rescue of Mutant P53. J.Am.Chem.Soc. V. 134 6810 2012.
ISSN: ISSN 0002-7863
PubMed: 22439615
DOI: 10.1021/JA301056A
Page generated: Sat Oct 26 19:13:49 2024
ISSN: ISSN 0002-7863
PubMed: 22439615
DOI: 10.1021/JA301056A
Last articles
As in 1C82As in 1BHL
As in 1D0C
As in 1BEH
As in 1B9D
As in 1B92
As in 1B9F
Ar in 7Q38
Ar in 6QAR
Ar in 6R1Q