Zinc »
PDB 3scl-3sje »
3shi »
Zinc in PDB 3shi: Crystal Structure of Human MMP1 Catalytic Domain at 2.2 A Resolution
Enzymatic activity of Crystal Structure of Human MMP1 Catalytic Domain at 2.2 A Resolution
All present enzymatic activity of Crystal Structure of Human MMP1 Catalytic Domain at 2.2 A Resolution:
3.4.24.7;
3.4.24.7;
Protein crystallography data
The structure of Crystal Structure of Human MMP1 Catalytic Domain at 2.2 A Resolution, PDB code: 3shi
was solved by
I.Bertini,
V.Calderone,
L.Cerofolini,
M.Fragai,
C.F.G.C.Geraldes,
P.Hermann,
C.Luchinat,
G.Parigi,
J.Teixeira,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 81.65 / 2.20 |
| Space group | C 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 147.694, 54.528, 94.905, 90.00, 120.69, 90.00 |
| R / Rfree (%) | 20.9 / 27.8 |
Other elements in 3shi:
The structure of Crystal Structure of Human MMP1 Catalytic Domain at 2.2 A Resolution also contains other interesting chemical elements:
| Calcium | (Ca) | 9 atoms |
Zinc Binding Sites:
The binding sites of Zinc atom in the Crystal Structure of Human MMP1 Catalytic Domain at 2.2 A Resolution
(pdb code 3shi). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 6 binding sites of Zinc where determined in the Crystal Structure of Human MMP1 Catalytic Domain at 2.2 A Resolution, PDB code: 3shi:
Jump to Zinc binding site number: 1; 2; 3; 4; 5; 6;
In total 6 binding sites of Zinc where determined in the Crystal Structure of Human MMP1 Catalytic Domain at 2.2 A Resolution, PDB code: 3shi:
Jump to Zinc binding site number: 1; 2; 3; 4; 5; 6;
Zinc binding site 1 out of 6 in 3shi
Go back to
Zinc binding site 1 out
of 6 in the Crystal Structure of Human MMP1 Catalytic Domain at 2.2 A Resolution
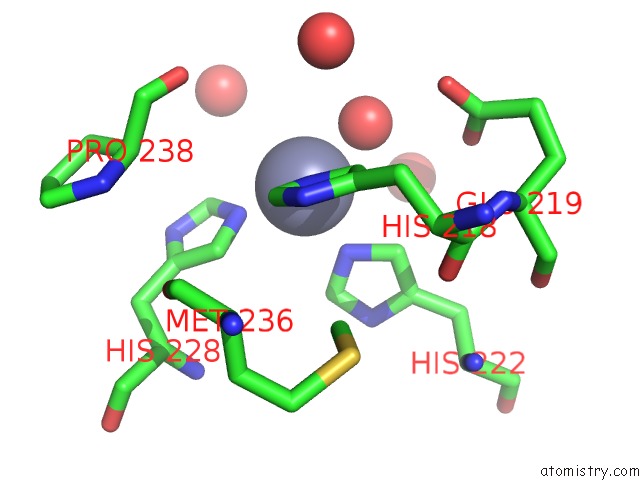
Mono view
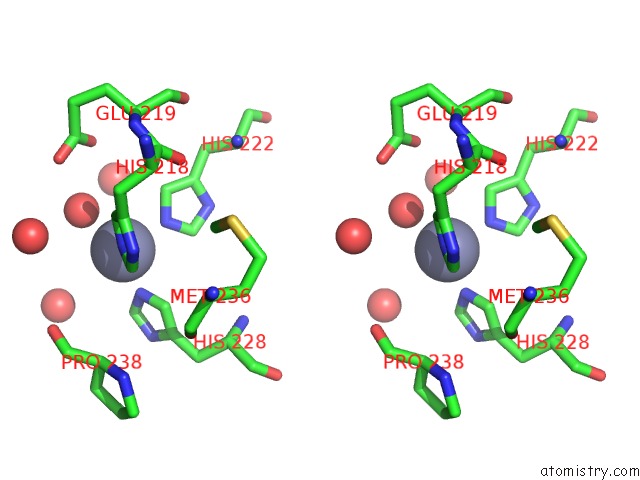
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Crystal Structure of Human MMP1 Catalytic Domain at 2.2 A Resolution within 5.0Å range:
|
Zinc binding site 2 out of 6 in 3shi
Go back to
Zinc binding site 2 out
of 6 in the Crystal Structure of Human MMP1 Catalytic Domain at 2.2 A Resolution
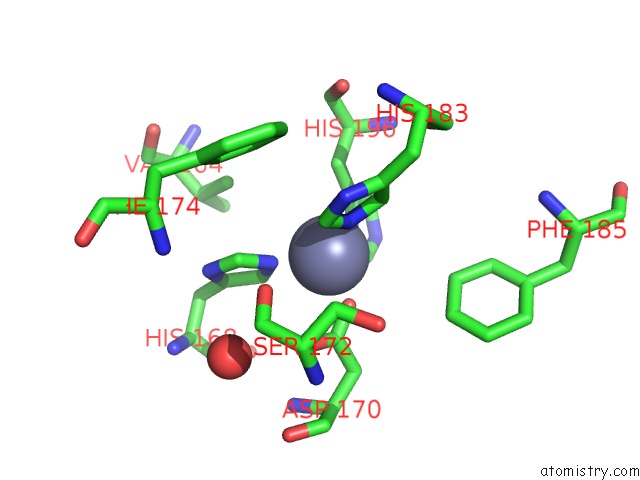
Mono view
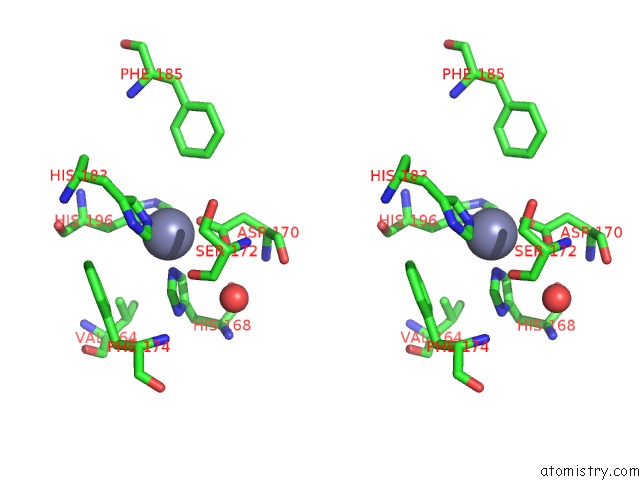
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of Crystal Structure of Human MMP1 Catalytic Domain at 2.2 A Resolution within 5.0Å range:
|
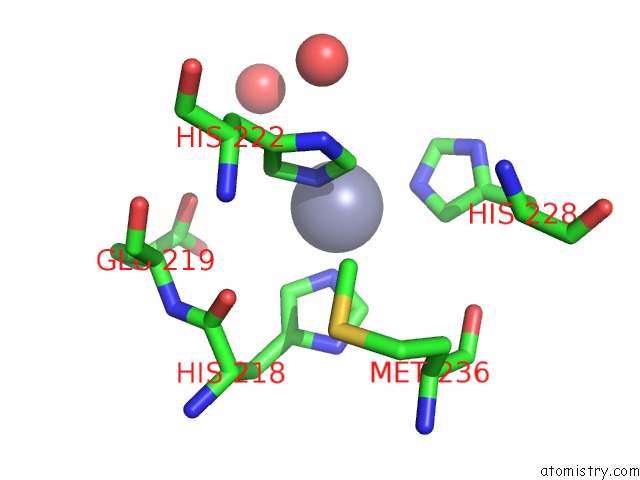
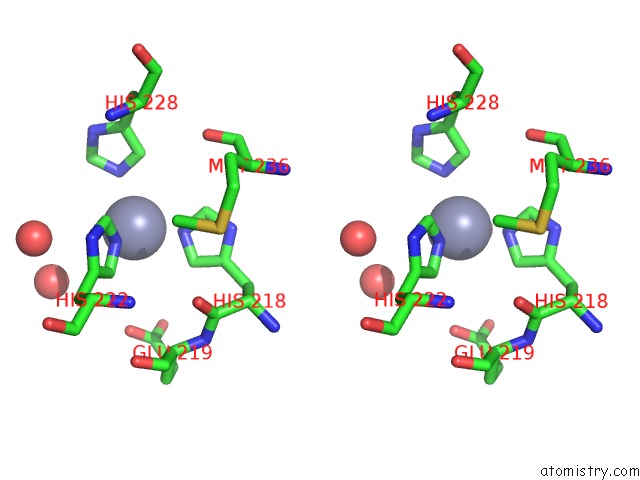
Zinc binding site 3 out of 6 in 3shi
Go back to
Zinc binding site 3 out
of 6 in the Crystal Structure of Human MMP1 Catalytic Domain at 2.2 A Resolution
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 3 of Crystal Structure of Human MMP1 Catalytic Domain at 2.2 A Resolution within 5.0Å range:
|
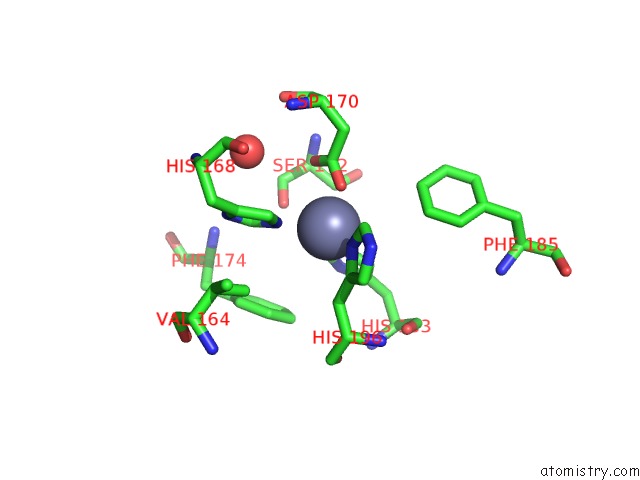
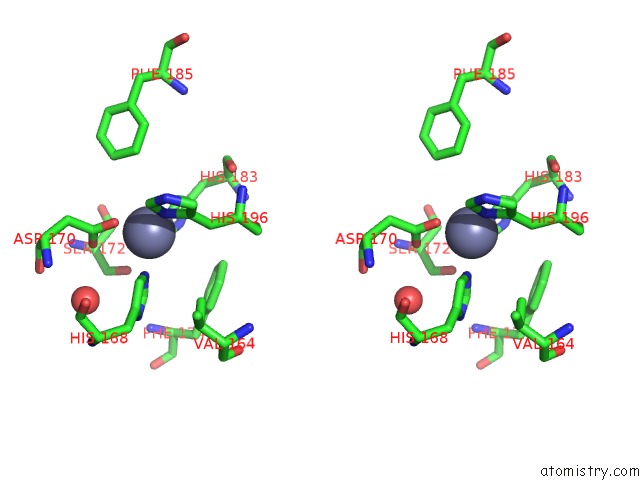
Zinc binding site 4 out of 6 in 3shi
Go back to
Zinc binding site 4 out
of 6 in the Crystal Structure of Human MMP1 Catalytic Domain at 2.2 A Resolution
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 4 of Crystal Structure of Human MMP1 Catalytic Domain at 2.2 A Resolution within 5.0Å range:
|
Zinc binding site 5 out of 6 in 3shi
Go back to
Zinc binding site 5 out
of 6 in the Crystal Structure of Human MMP1 Catalytic Domain at 2.2 A Resolution
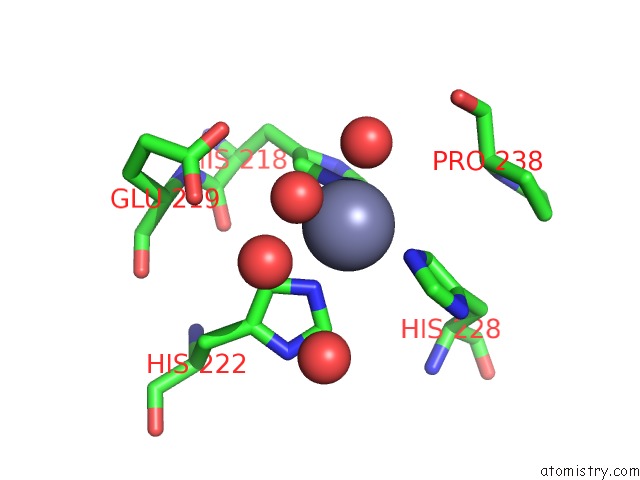
Mono view
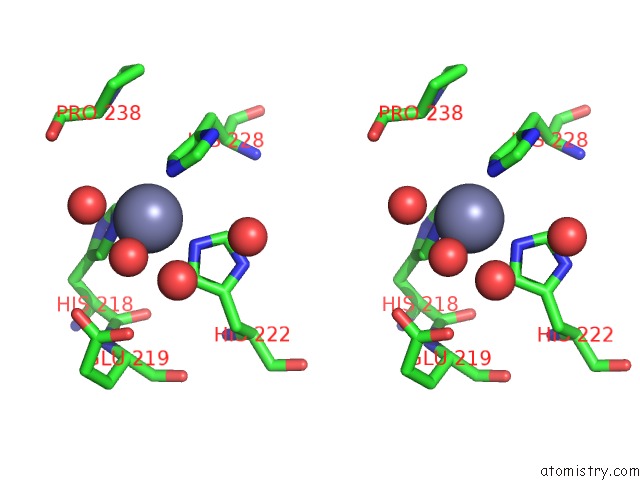
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 5 of Crystal Structure of Human MMP1 Catalytic Domain at 2.2 A Resolution within 5.0Å range:
|
Zinc binding site 6 out of 6 in 3shi
Go back to
Zinc binding site 6 out
of 6 in the Crystal Structure of Human MMP1 Catalytic Domain at 2.2 A Resolution
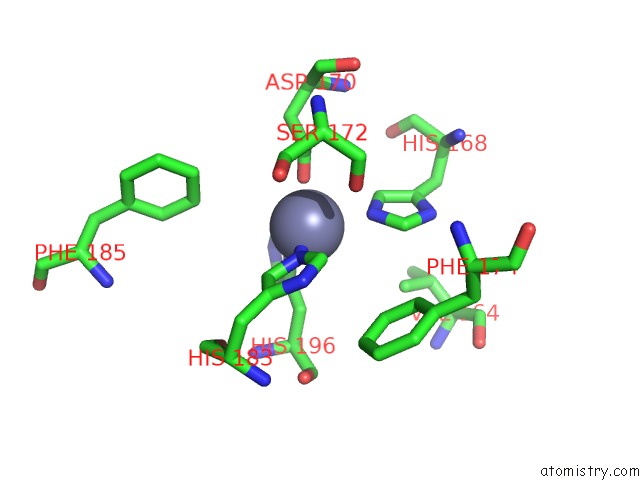
Mono view
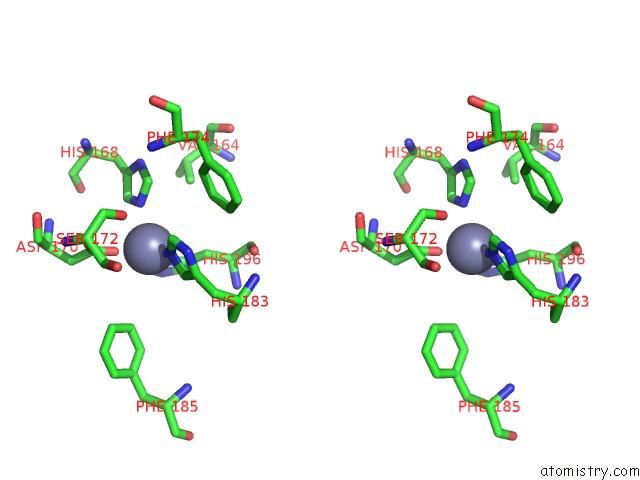
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 6 of Crystal Structure of Human MMP1 Catalytic Domain at 2.2 A Resolution within 5.0Å range:
|
Reference:
I.Bertini,
V.Calderone,
L.Cerofolini,
M.Fragai,
C.F.Geraldes,
P.Hermann,
C.Luchinat,
G.Parigi,
J.M.Teixeira.
The Catalytic Domain of Mmp-1 Studied Through Tagged Lanthanides. Febs Lett. V. 586 557 2012.
ISSN: ISSN 0014-5793
PubMed: 21945315
DOI: 10.1016/J.FEBSLET.2011.09.020
Page generated: Wed Aug 20 14:02:35 2025
ISSN: ISSN 0014-5793
PubMed: 21945315
DOI: 10.1016/J.FEBSLET.2011.09.020
Last articles
Zn in 4JLWZn in 4JOM
Zn in 4JMY
Zn in 4JLZ
Zn in 4JKR
Zn in 4JLX
Zn in 4JK2
Zn in 4JK1
Zn in 4JJI
Zn in 4JIW