Zinc »
PDB 3mru-3n3j »
3mtw »
Zinc in PDB 3mtw: Crystal Structure of L-Lysine, L-Arginine Carboxypeptidase CC2672 From Caulobacter Crescentus CB15 Complexed with N-Methyl Phosphonate Derivative of L-Arginine
Protein crystallography data
The structure of Crystal Structure of L-Lysine, L-Arginine Carboxypeptidase CC2672 From Caulobacter Crescentus CB15 Complexed with N-Methyl Phosphonate Derivative of L-Arginine, PDB code: 3mtw
was solved by
A.A.Fedorov,
E.V.Fedorov,
D.F.Xiang,
F.M.Raushel,
S.C.Almo,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 34.35 / 1.70 |
| Space group | I 4 3 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 200.315, 200.315, 200.315, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 18 / 20.3 |
Zinc Binding Sites:
The binding sites of Zinc atom in the Crystal Structure of L-Lysine, L-Arginine Carboxypeptidase CC2672 From Caulobacter Crescentus CB15 Complexed with N-Methyl Phosphonate Derivative of L-Arginine
(pdb code 3mtw). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 2 binding sites of Zinc where determined in the Crystal Structure of L-Lysine, L-Arginine Carboxypeptidase CC2672 From Caulobacter Crescentus CB15 Complexed with N-Methyl Phosphonate Derivative of L-Arginine, PDB code: 3mtw:
Jump to Zinc binding site number: 1; 2;
In total 2 binding sites of Zinc where determined in the Crystal Structure of L-Lysine, L-Arginine Carboxypeptidase CC2672 From Caulobacter Crescentus CB15 Complexed with N-Methyl Phosphonate Derivative of L-Arginine, PDB code: 3mtw:
Jump to Zinc binding site number: 1; 2;
Zinc binding site 1 out of 2 in 3mtw
Go back to
Zinc binding site 1 out
of 2 in the Crystal Structure of L-Lysine, L-Arginine Carboxypeptidase CC2672 From Caulobacter Crescentus CB15 Complexed with N-Methyl Phosphonate Derivative of L-Arginine
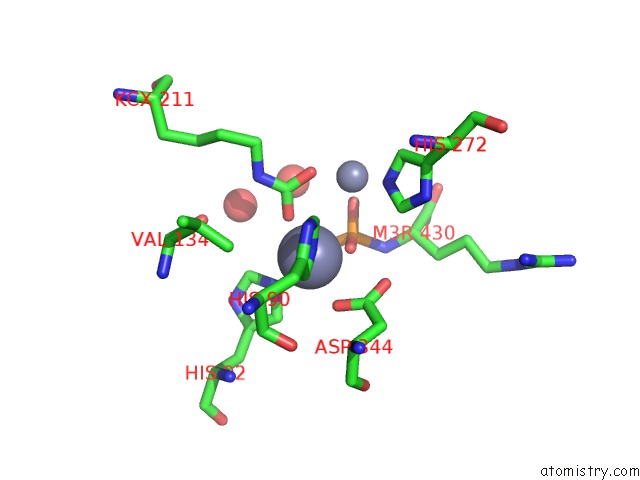
Mono view
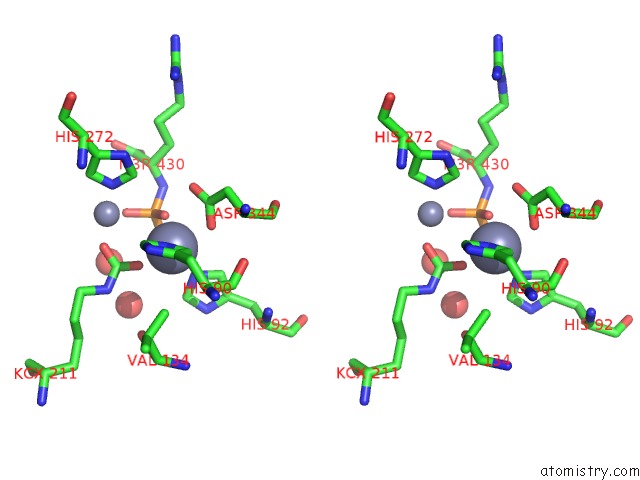
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Crystal Structure of L-Lysine, L-Arginine Carboxypeptidase CC2672 From Caulobacter Crescentus CB15 Complexed with N-Methyl Phosphonate Derivative of L-Arginine within 5.0Å range:
|
Zinc binding site 2 out of 2 in 3mtw
Go back to
Zinc binding site 2 out
of 2 in the Crystal Structure of L-Lysine, L-Arginine Carboxypeptidase CC2672 From Caulobacter Crescentus CB15 Complexed with N-Methyl Phosphonate Derivative of L-Arginine
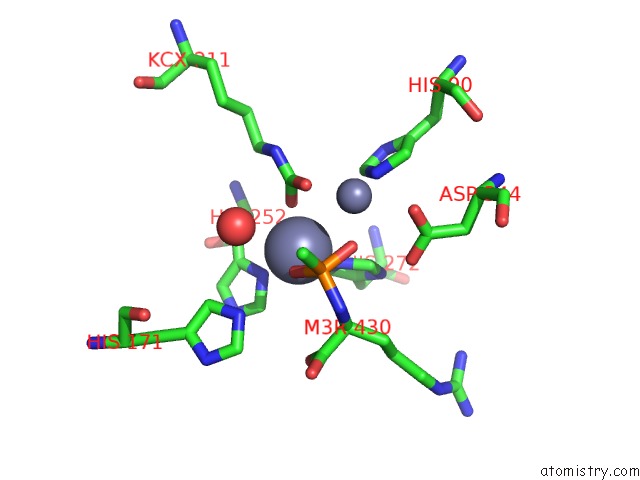
Mono view
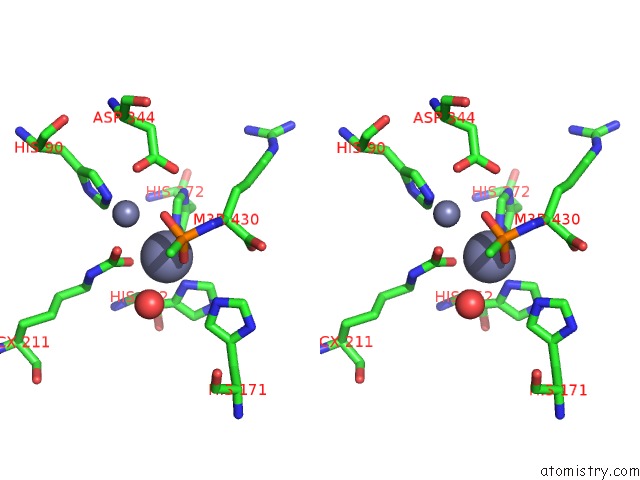
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of Crystal Structure of L-Lysine, L-Arginine Carboxypeptidase CC2672 From Caulobacter Crescentus CB15 Complexed with N-Methyl Phosphonate Derivative of L-Arginine within 5.0Å range:
|
Reference:
D.F.Xiang,
Y.Patskovsky,
C.Xu,
A.A.Fedorov,
E.V.Fedorov,
A.A.Sisco,
J.M.Sauder,
S.K.Burley,
S.C.Almo,
F.M.Raushel.
Functional Identification and Structure Determination of Two Novel Prolidases From COG1228 in the Amidohydrolase Superfamily Biochemistry V. 49 6791 2010.
ISSN: ISSN 0006-2960
PubMed: 20604542
DOI: 10.1021/BI100897U
Page generated: Sat Oct 26 09:48:31 2024
ISSN: ISSN 0006-2960
PubMed: 20604542
DOI: 10.1021/BI100897U
Last articles
As in 4CI1As in 4CU1
As in 4CU0
As in 4CTZ
As in 4CTY
As in 4B9K
As in 4B09
As in 4CFT
As in 4CAR
As in 4BKT