Zinc »
PDB 3m1q-3m6r »
3m1w »
Zinc in PDB 3m1w: Carbonic Anhyrdase II Mutant W5CH64C with Closed Disulfide Bond in Complex with Sulfate
Enzymatic activity of Carbonic Anhyrdase II Mutant W5CH64C with Closed Disulfide Bond in Complex with Sulfate
All present enzymatic activity of Carbonic Anhyrdase II Mutant W5CH64C with Closed Disulfide Bond in Complex with Sulfate:
4.2.1.1;
4.2.1.1;
Protein crystallography data
The structure of Carbonic Anhyrdase II Mutant W5CH64C with Closed Disulfide Bond in Complex with Sulfate, PDB code: 3m1w
was solved by
J.Schulze Wischeler,
A.Heine,
G.Klebe,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 10.00 / 1.38 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 42.300, 41.500, 72.200, 90.00, 104.40, 90.00 |
| R / Rfree (%) | 13.1 / 17.5 |
Zinc Binding Sites:
The binding sites of Zinc atom in the Carbonic Anhyrdase II Mutant W5CH64C with Closed Disulfide Bond in Complex with Sulfate
(pdb code 3m1w). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total only one binding site of Zinc was determined in the Carbonic Anhyrdase II Mutant W5CH64C with Closed Disulfide Bond in Complex with Sulfate, PDB code: 3m1w:
In total only one binding site of Zinc was determined in the Carbonic Anhyrdase II Mutant W5CH64C with Closed Disulfide Bond in Complex with Sulfate, PDB code: 3m1w:
Zinc binding site 1 out of 1 in 3m1w
Go back to
Zinc binding site 1 out
of 1 in the Carbonic Anhyrdase II Mutant W5CH64C with Closed Disulfide Bond in Complex with Sulfate
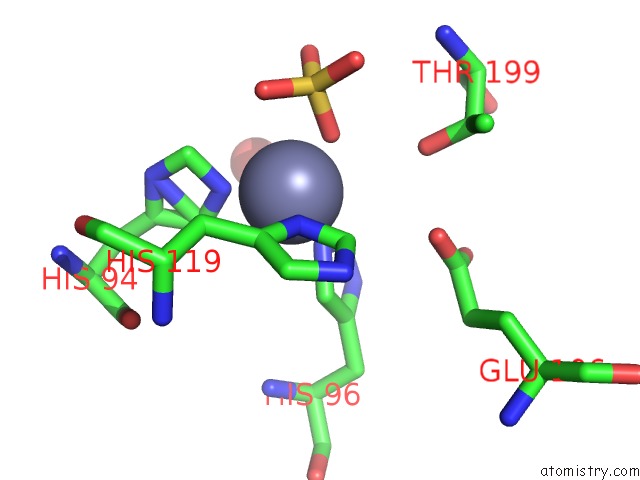
Mono view
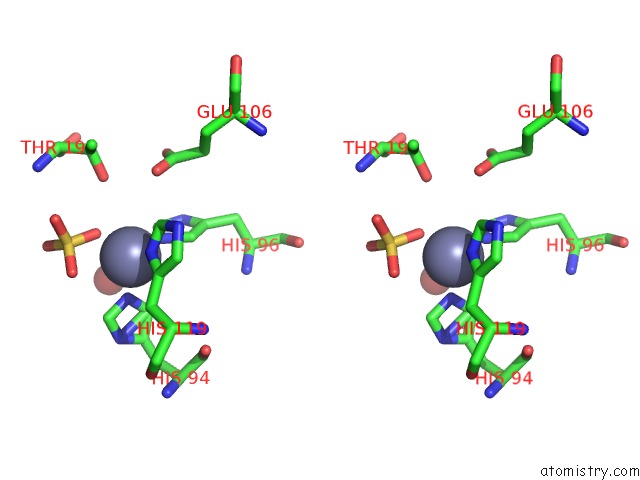
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Carbonic Anhyrdase II Mutant W5CH64C with Closed Disulfide Bond in Complex with Sulfate within 5.0Å range:
|
Reference:
J.Schulze Wischeler,
N.U.Sandner,
A.Heine,
G.Klebe.
Mutational Study on Carbonic Anhydrase II To Be Published.
Page generated: Wed Aug 20 11:35:04 2025
Last articles
Zn in 4HEYZn in 4HDT
Zn in 4HEV
Zn in 4HEW
Zn in 4HE2
Zn in 4HDH
Zn in 4HDG
Zn in 4HCG
Zn in 4HDA
Zn in 4HD8