Zinc »
PDB 2yu8-2z56 »
2z56 »
Zinc in PDB 2z56: Crystal Structure of G56S-Propeptide:S324A-Subtilisin Complex
Enzymatic activity of Crystal Structure of G56S-Propeptide:S324A-Subtilisin Complex
All present enzymatic activity of Crystal Structure of G56S-Propeptide:S324A-Subtilisin Complex:
3.4.21.62;
3.4.21.62;
Protein crystallography data
The structure of Crystal Structure of G56S-Propeptide:S324A-Subtilisin Complex, PDB code: 2z56
was solved by
M.A.Pulido,
S.Tanaka,
C.Sringiew,
D.J.You,
H.Matsumura,
Y.Koga,
K.Takano,
S.Kanaya,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 50.00 / 1.90 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 64.518, 67.463, 74.046, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 18.9 / 20.9 |
Other elements in 2z56:
The structure of Crystal Structure of G56S-Propeptide:S324A-Subtilisin Complex also contains other interesting chemical elements:
| Calcium | (Ca) | 7 atoms |
Zinc Binding Sites:
The binding sites of Zinc atom in the Crystal Structure of G56S-Propeptide:S324A-Subtilisin Complex
(pdb code 2z56). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total only one binding site of Zinc was determined in the Crystal Structure of G56S-Propeptide:S324A-Subtilisin Complex, PDB code: 2z56:
In total only one binding site of Zinc was determined in the Crystal Structure of G56S-Propeptide:S324A-Subtilisin Complex, PDB code: 2z56:
Zinc binding site 1 out of 1 in 2z56
Go back to
Zinc binding site 1 out
of 1 in the Crystal Structure of G56S-Propeptide:S324A-Subtilisin Complex
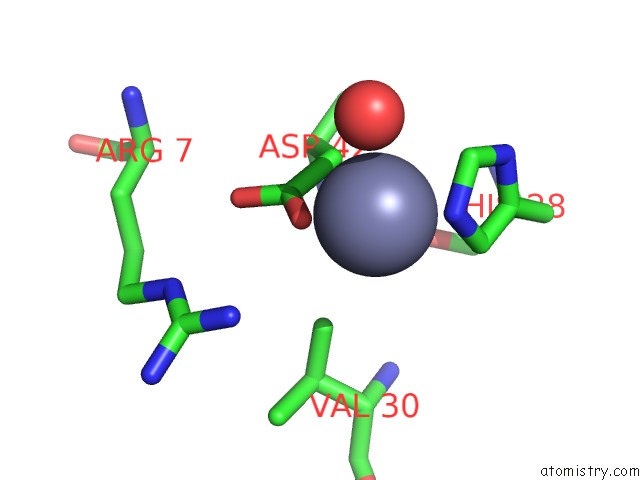
Mono view
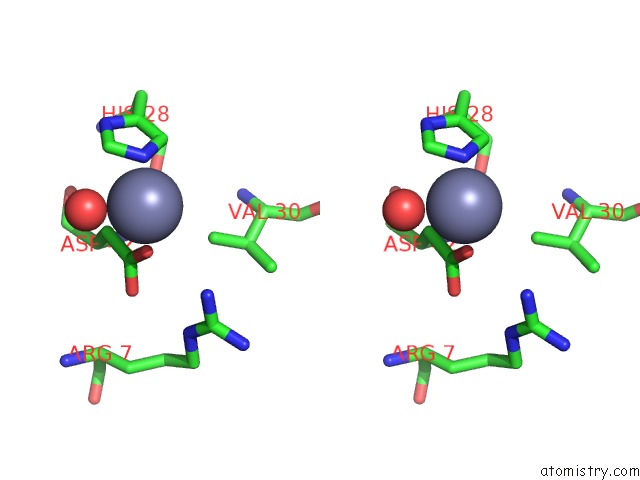
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Crystal Structure of G56S-Propeptide:S324A-Subtilisin Complex within 5.0Å range:
|
Reference:
M.A.Pulido,
S.Tanaka,
C.Sringiew,
D.J.You,
H.Matsumura,
Y.Koga,
K.Takano,
S.Kanaya.
Requirement of Left-Handed Glycine Residue For High Stability of the Tk-Subtilisin Propeptide As Revealed By Mutational and Crystallographic Analyses J.Mol.Biol. V. 374 1359 2007.
ISSN: ISSN 0022-2836
PubMed: 17988685
DOI: 10.1016/J.JMB.2007.10.030
Page generated: Wed Aug 20 07:22:16 2025
ISSN: ISSN 0022-2836
PubMed: 17988685
DOI: 10.1016/J.JMB.2007.10.030
Last articles
Zn in 3S0NZn in 3RZV
Zn in 3RYM
Zn in 3RZA
Zn in 3RZ8
Zn in 3RZ7
Zn in 3RZ5
Zn in 3RZ1
Zn in 3RZ0
Zn in 3RYS