Zinc »
PDB 2xy9-2y7g »
2y28 »
Zinc in PDB 2y28: Crystal Structure of Se-Met Ampd Derivative
Enzymatic activity of Crystal Structure of Se-Met Ampd Derivative
All present enzymatic activity of Crystal Structure of Se-Met Ampd Derivative:
3.5.1.28;
3.5.1.28;
Protein crystallography data
The structure of Crystal Structure of Se-Met Ampd Derivative, PDB code: 2y28
was solved by
C.Carrasco-Lopez,
A.Rojas-Altuve,
W.Zhang,
D.Hesek,
M.Lee,
S.Barbe,
I.Andre,
N.Silva-Martin,
M.Martinez-Ripoll,
S.Mobashery,
J.A.Hermoso,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 58.624 / 1.80 |
| Space group | P 32 |
| Cell size a, b, c (Å), α, β, γ (°) | 67.693, 67.693, 92.681, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 17.8 / 23.18 |
Zinc Binding Sites:
The binding sites of Zinc atom in the Crystal Structure of Se-Met Ampd Derivative
(pdb code 2y28). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 3 binding sites of Zinc where determined in the Crystal Structure of Se-Met Ampd Derivative, PDB code: 2y28:
Jump to Zinc binding site number: 1; 2; 3;
In total 3 binding sites of Zinc where determined in the Crystal Structure of Se-Met Ampd Derivative, PDB code: 2y28:
Jump to Zinc binding site number: 1; 2; 3;
Zinc binding site 1 out of 3 in 2y28
Go back to
Zinc binding site 1 out
of 3 in the Crystal Structure of Se-Met Ampd Derivative

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Crystal Structure of Se-Met Ampd Derivative within 5.0Å range:
|
Zinc binding site 2 out of 3 in 2y28
Go back to
Zinc binding site 2 out
of 3 in the Crystal Structure of Se-Met Ampd Derivative

Mono view
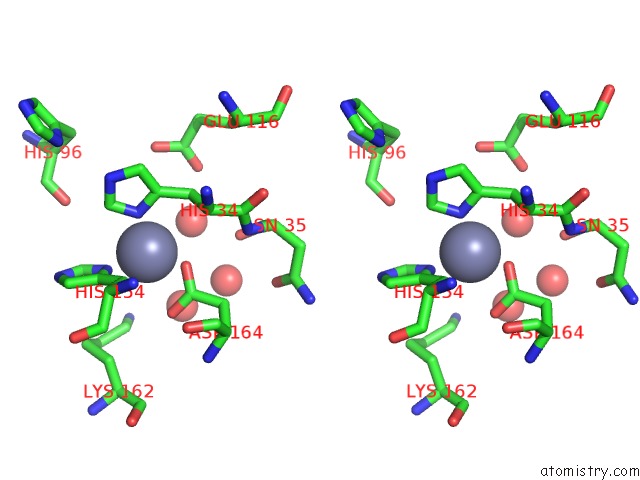
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of Crystal Structure of Se-Met Ampd Derivative within 5.0Å range:
|
Zinc binding site 3 out of 3 in 2y28
Go back to
Zinc binding site 3 out
of 3 in the Crystal Structure of Se-Met Ampd Derivative

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 3 of Crystal Structure of Se-Met Ampd Derivative within 5.0Å range:
|
Reference:
C.Carrasco-Lopez,
A.Rojas-Altuve,
W.Zhang,
D.Hesek,
M.Lee,
S.Barbe,
I.Andre,
P.Ferrer,
N.Silva-Martin,
G.R.Castro,
M.Martinez-Ripoll,
S.Mobashery,
J.A.Hermoso.
Crystal Structures of Bacterial Peptidoglycan Amidase Ampd and An Unprecedented Activation Mechanism. J.Biol.Chem. V. 286 31714 2011.
ISSN: ISSN 0021-9258
PubMed: 21775432
DOI: 10.1074/JBC.M111.264366
Page generated: Wed Aug 20 06:58:44 2025
ISSN: ISSN 0021-9258
PubMed: 21775432
DOI: 10.1074/JBC.M111.264366
Last articles
Zn in 3QLNZn in 3QMC
Zn in 3QMB
Zn in 3QLC
Zn in 3QLA
Zn in 3QL9
Zn in 3QJ5
Zn in 3QG6
Zn in 3QJX
Zn in 3QJ0