Zinc »
PDB 2naa-2nwy »
2nvb »
Zinc in PDB 2nvb: Contribution of PRO275 to the Thermostability of the Alcohol Dehydrogenases (Adhs)
Enzymatic activity of Contribution of PRO275 to the Thermostability of the Alcohol Dehydrogenases (Adhs)
All present enzymatic activity of Contribution of PRO275 to the Thermostability of the Alcohol Dehydrogenases (Adhs):
1.1.1.2;
1.1.1.2;
Protein crystallography data
The structure of Contribution of PRO275 to the Thermostability of the Alcohol Dehydrogenases (Adhs), PDB code: 2nvb
was solved by
E.Goihberg,
S.Tel-Or,
M.Peretz,
F.Frolow,
O.Dym,
Y.Burstein,
Israel Structural Proteomics Center (Ispc),
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 39.60 / 2.80 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 79.598, 125.008, 167.111, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 21.7 / 27.8 |
Zinc Binding Sites:
The binding sites of Zinc atom in the Contribution of PRO275 to the Thermostability of the Alcohol Dehydrogenases (Adhs)
(pdb code 2nvb). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 4 binding sites of Zinc where determined in the Contribution of PRO275 to the Thermostability of the Alcohol Dehydrogenases (Adhs), PDB code: 2nvb:
Jump to Zinc binding site number: 1; 2; 3; 4;
In total 4 binding sites of Zinc where determined in the Contribution of PRO275 to the Thermostability of the Alcohol Dehydrogenases (Adhs), PDB code: 2nvb:
Jump to Zinc binding site number: 1; 2; 3; 4;
Zinc binding site 1 out of 4 in 2nvb
Go back to
Zinc binding site 1 out
of 4 in the Contribution of PRO275 to the Thermostability of the Alcohol Dehydrogenases (Adhs)
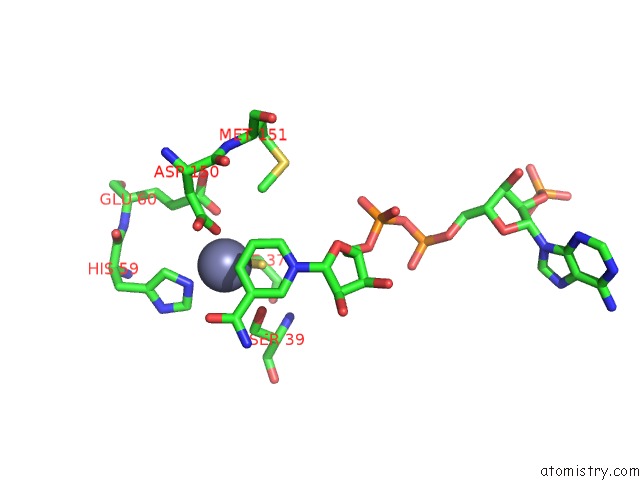
Mono view
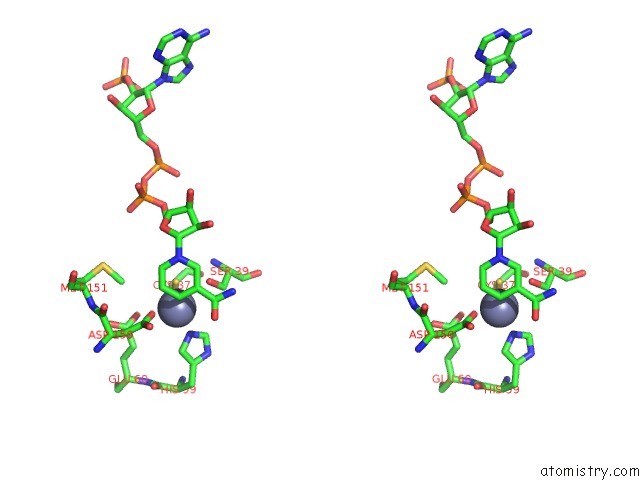
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Contribution of PRO275 to the Thermostability of the Alcohol Dehydrogenases (Adhs) within 5.0Å range:
|
Zinc binding site 2 out of 4 in 2nvb
Go back to
Zinc binding site 2 out
of 4 in the Contribution of PRO275 to the Thermostability of the Alcohol Dehydrogenases (Adhs)
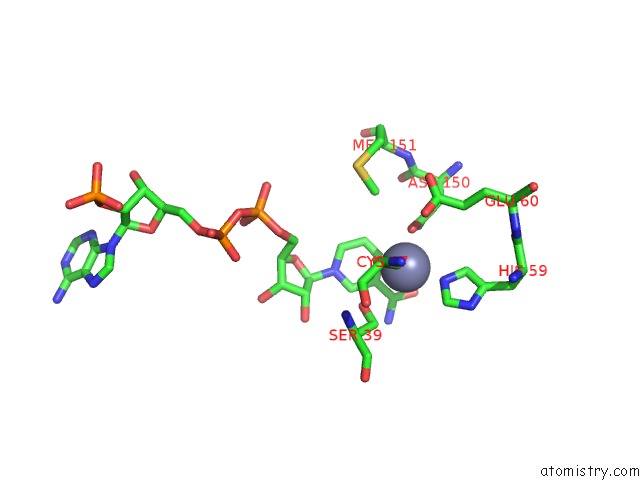
Mono view
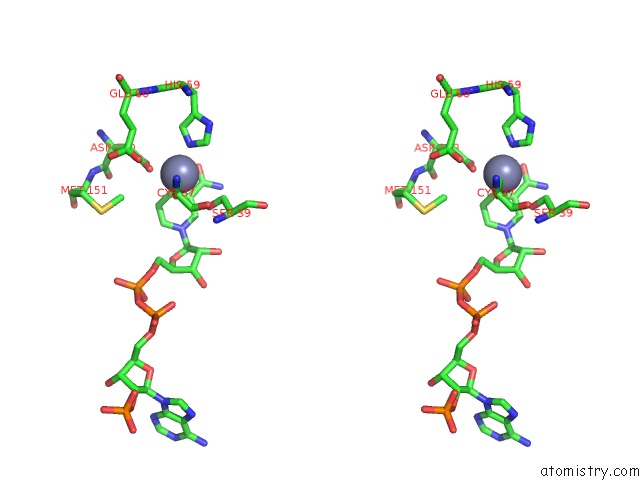
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of Contribution of PRO275 to the Thermostability of the Alcohol Dehydrogenases (Adhs) within 5.0Å range:
|
Zinc binding site 3 out of 4 in 2nvb
Go back to
Zinc binding site 3 out
of 4 in the Contribution of PRO275 to the Thermostability of the Alcohol Dehydrogenases (Adhs)
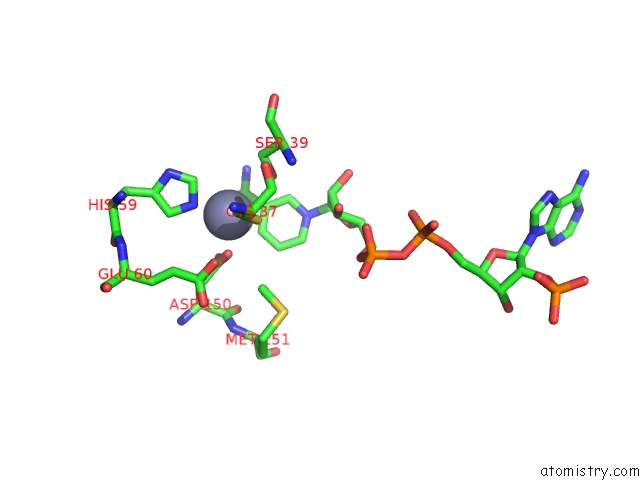
Mono view
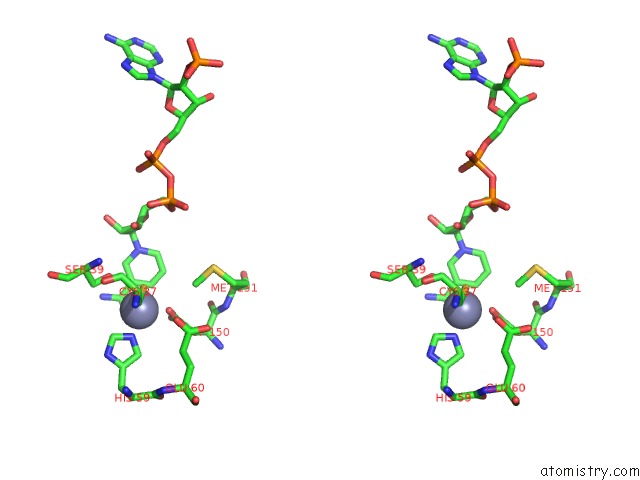
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 3 of Contribution of PRO275 to the Thermostability of the Alcohol Dehydrogenases (Adhs) within 5.0Å range:
|
Zinc binding site 4 out of 4 in 2nvb
Go back to
Zinc binding site 4 out
of 4 in the Contribution of PRO275 to the Thermostability of the Alcohol Dehydrogenases (Adhs)
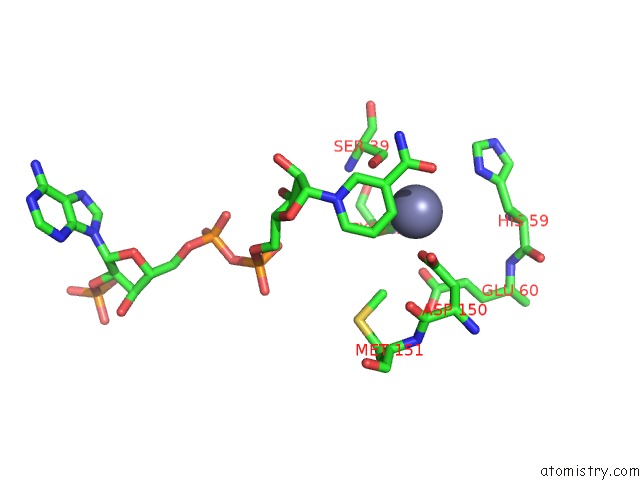
Mono view
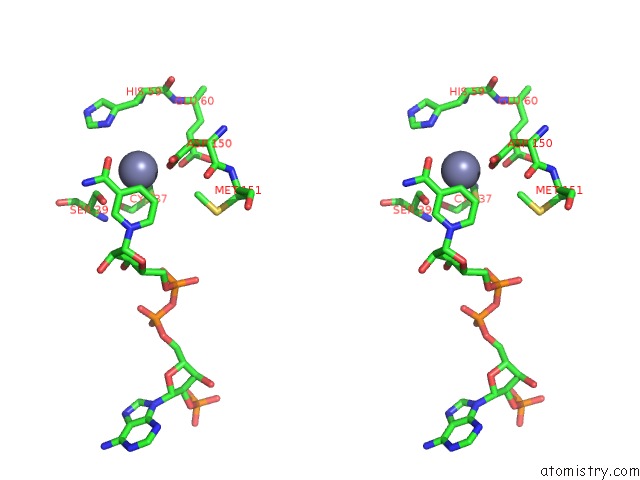
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 4 of Contribution of PRO275 to the Thermostability of the Alcohol Dehydrogenases (Adhs) within 5.0Å range:
|
Reference:
E.Goihberg,
O.Dym,
S.Tel-Or,
L.Shimon,
F.Frolow,
M.Peretz,
Y.Burstein.
Thermal Stabilization of the Protozoan Entamoeba Histolytica Alcohol Dehydrogenase By A Single Proline Substitution Proteins V. 72 711 2008.
ISSN: ISSN 0887-3585
PubMed: 18260103
DOI: 10.1002/PROT.21946
Page generated: Thu Oct 17 02:17:37 2024
ISSN: ISSN 0887-3585
PubMed: 18260103
DOI: 10.1002/PROT.21946
Last articles
Al in 8SG9Al in 8SFF
Al in 8SG8
Al in 8R1A
Al in 8Q75
Al in 8OIE
Al in 8OX6
Al in 8OX5
Al in 8OP8
Al in 8OP6