Zinc »
PDB 2mmi-2n9p »
2mze »
Zinc in PDB 2mze: uc(Nmr) Solution Structure of the Pro Form of Human Matrilysin (Prommp-7)
Enzymatic activity of uc(Nmr) Solution Structure of the Pro Form of Human Matrilysin (Prommp-7)
All present enzymatic activity of uc(Nmr) Solution Structure of the Pro Form of Human Matrilysin (Prommp-7):
3.4.24.23;
3.4.24.23;
Other elements in 2mze:
The structure of uc(Nmr) Solution Structure of the Pro Form of Human Matrilysin (Prommp-7) also contains other interesting chemical elements:
| Calcium | (Ca) | 40 atoms |
Zinc Binding Sites:
The binding sites of Zinc atom in the uc(Nmr) Solution Structure of the Pro Form of Human Matrilysin (Prommp-7)
(pdb code 2mze). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 2 binding sites of Zinc where determined in the uc(Nmr) Solution Structure of the Pro Form of Human Matrilysin (Prommp-7), PDB code: 2mze:
Jump to Zinc binding site number: 1; 2;
In total 2 binding sites of Zinc where determined in the uc(Nmr) Solution Structure of the Pro Form of Human Matrilysin (Prommp-7), PDB code: 2mze:
Jump to Zinc binding site number: 1; 2;
Zinc binding site 1 out of 2 in 2mze
Go back to
Zinc binding site 1 out
of 2 in the uc(Nmr) Solution Structure of the Pro Form of Human Matrilysin (Prommp-7)
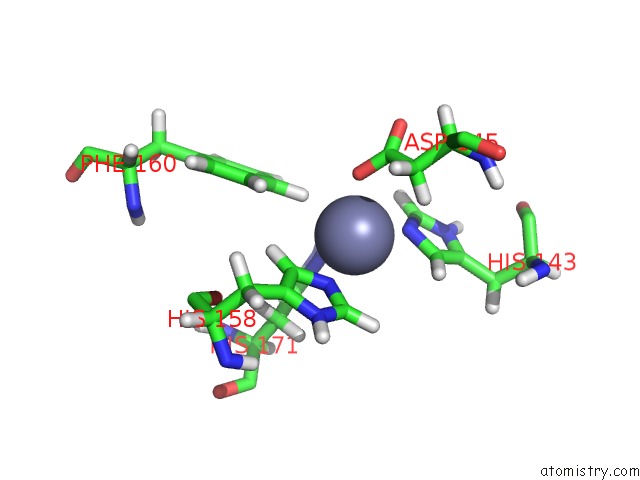
Mono view
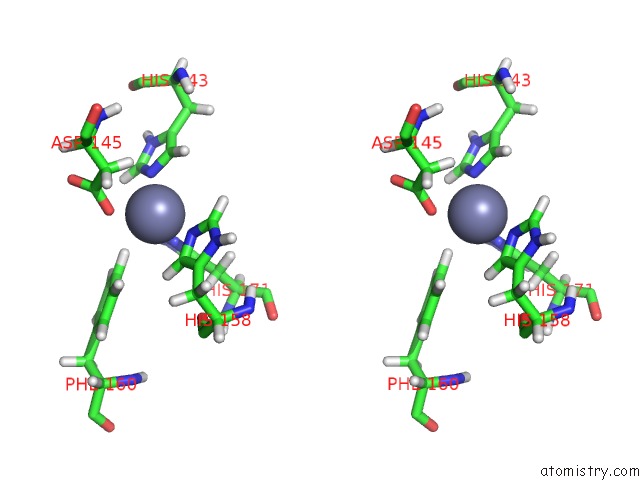
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of uc(Nmr) Solution Structure of the Pro Form of Human Matrilysin (Prommp-7) within 5.0Å range:
|
Zinc binding site 2 out of 2 in 2mze
Go back to
Zinc binding site 2 out
of 2 in the uc(Nmr) Solution Structure of the Pro Form of Human Matrilysin (Prommp-7)
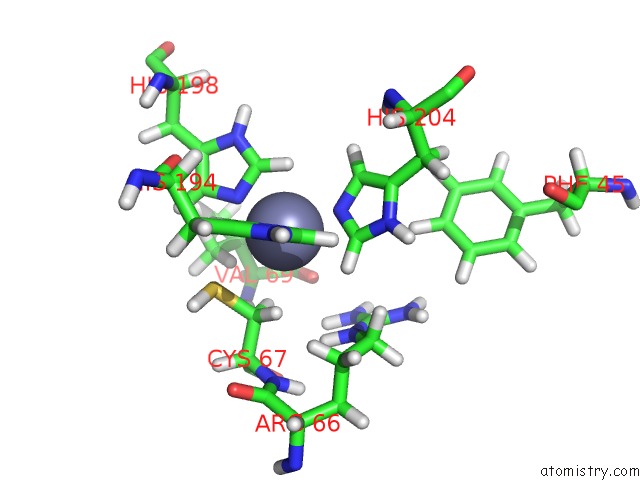
Mono view
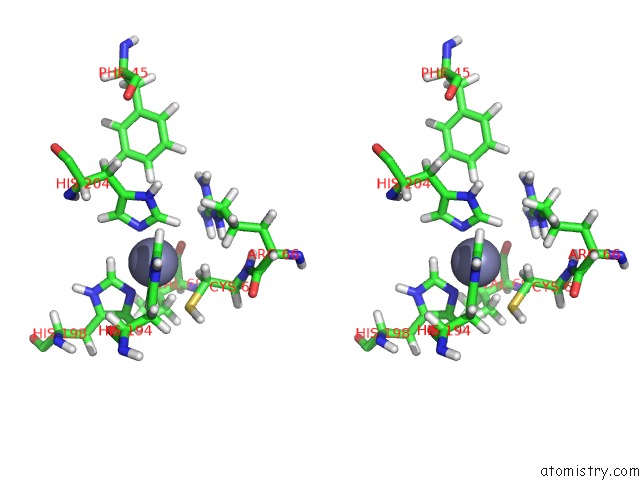
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of uc(Nmr) Solution Structure of the Pro Form of Human Matrilysin (Prommp-7) within 5.0Å range:
|
Reference:
S.H.Prior,
Y.G.Fulcher,
R.K.Koppisetti,
A.Jurkevich,
S.R.Van Doren.
Charge-Triggered Membrane Insertion of Matrix Metalloproteinase-7, Supporter of Innate Immunity and Tumors. Structure V. 23 2099 2015.
ISSN: ISSN 0969-2126
PubMed: 26439767
DOI: 10.1016/J.STR.2015.08.013
Page generated: Thu Oct 17 02:10:01 2024
ISSN: ISSN 0969-2126
PubMed: 26439767
DOI: 10.1016/J.STR.2015.08.013
Last articles
Ag in 7XLVAg in 8DX7
Ag in 8DX6
Ag in 7XKM
Ag in 8DX5
Ag in 8DX1
Ag in 8C2Q
Ag in 7WAA
Ag in 7TMJ
Ag in 7TGD