Zinc »
PDB 2geh-2gzg »
2gfo »
Zinc in PDB 2gfo: Structure of the Catalytic Domain of Human Ubiquitin Carboxyl-Terminal Hydrolase 8
Enzymatic activity of Structure of the Catalytic Domain of Human Ubiquitin Carboxyl-Terminal Hydrolase 8
All present enzymatic activity of Structure of the Catalytic Domain of Human Ubiquitin Carboxyl-Terminal Hydrolase 8:
3.1.2.15;
3.1.2.15;
Protein crystallography data
The structure of Structure of the Catalytic Domain of Human Ubiquitin Carboxyl-Terminal Hydrolase 8, PDB code: 2gfo
was solved by
J.R.Walker,
G.V.Avvakumov,
S.Xue,
E.M.Newman,
P.J.Finerty Jr.,
C.Butler-Cole,
J.Weigelt,
M.Sundstrom,
C.Arrowsmith,
A.Edwards,
A.Bochkarev,
S.Dhe-Paganon,
Structural Genomics Consortium (Sgc),
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 18.20 / 2.00 |
| Space group | P 61 |
| Cell size a, b, c (Å), α, β, γ (°) | 67.173, 67.173, 194.458, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 16.8 / 21 |
Zinc Binding Sites:
The binding sites of Zinc atom in the Structure of the Catalytic Domain of Human Ubiquitin Carboxyl-Terminal Hydrolase 8
(pdb code 2gfo). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total only one binding site of Zinc was determined in the Structure of the Catalytic Domain of Human Ubiquitin Carboxyl-Terminal Hydrolase 8, PDB code: 2gfo:
In total only one binding site of Zinc was determined in the Structure of the Catalytic Domain of Human Ubiquitin Carboxyl-Terminal Hydrolase 8, PDB code: 2gfo:
Zinc binding site 1 out of 1 in 2gfo
Go back to
Zinc binding site 1 out
of 1 in the Structure of the Catalytic Domain of Human Ubiquitin Carboxyl-Terminal Hydrolase 8
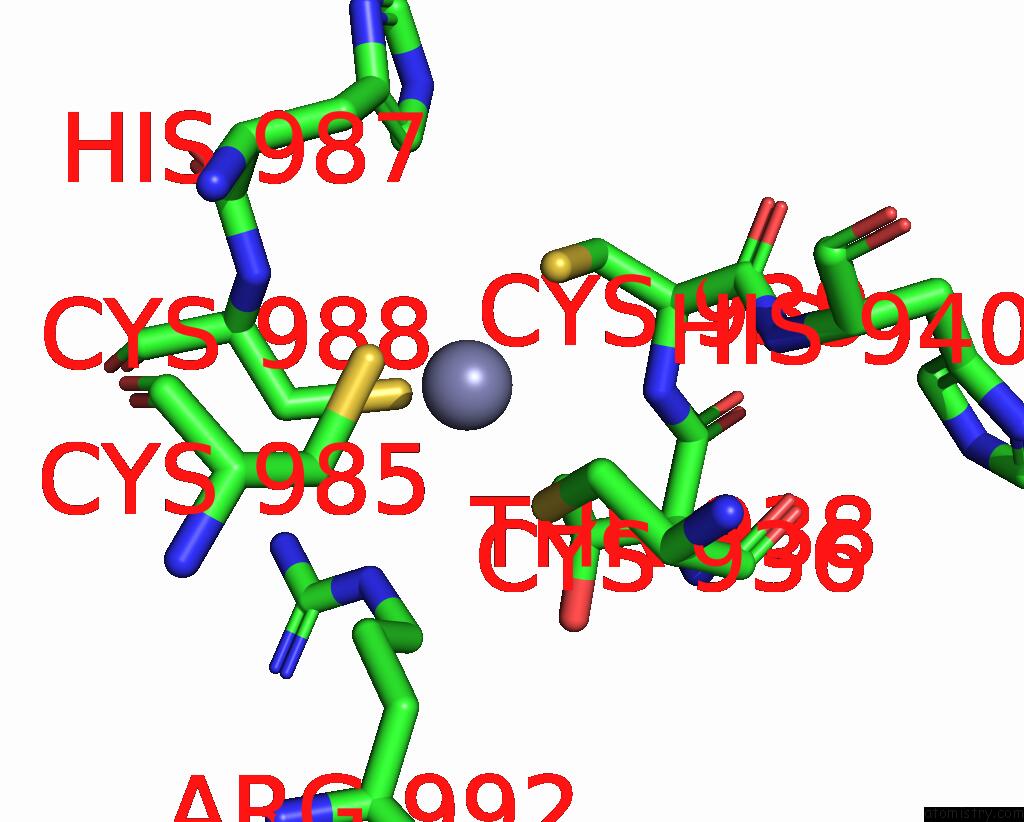
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Structure of the Catalytic Domain of Human Ubiquitin Carboxyl-Terminal Hydrolase 8 within 5.0Å range:
|
Reference:
G.V.Avvakumov,
J.R.Walker,
S.Xue,
P.J.Finerty Jr.,
F.Mackenzie,
E.M.Newman,
S.Dhe-Paganon.
Amino-Terminal Dimerization, NRDP1-Rhodanese Interaction, and Inhibited Catalytic Domain Conformation of the Ubiquitin-Specific Protease 8 (USP8). J.Biol.Chem. V. 281 38061 2006.
ISSN: ISSN 0021-9258
PubMed: 17035239
DOI: 10.1074/JBC.M606704200
Page generated: Thu Oct 17 00:18:30 2024
ISSN: ISSN 0021-9258
PubMed: 17035239
DOI: 10.1074/JBC.M606704200
Last articles
Ag in 7XLVAg in 8DX7
Ag in 8DX6
Ag in 7XKM
Ag in 8DX5
Ag in 8DX1
Ag in 8C2Q
Ag in 7WAA
Ag in 7TMJ
Ag in 7TGD