Zinc »
PDB 1tbt-1to4 »
1teu »
Zinc in PDB 1teu: Effect of Shuttle Location and pH Environment on H+ Transfer in Human Carbonic Anhydrase II
Enzymatic activity of Effect of Shuttle Location and pH Environment on H+ Transfer in Human Carbonic Anhydrase II
All present enzymatic activity of Effect of Shuttle Location and pH Environment on H+ Transfer in Human Carbonic Anhydrase II:
4.2.1.1;
4.2.1.1;
Protein crystallography data
The structure of Effect of Shuttle Location and pH Environment on H+ Transfer in Human Carbonic Anhydrase II, PDB code: 1teu
was solved by
Z.Fisher,
J.A.Hernandez Prada,
C.K.Tu,
D.Duda,
C.Yoshioka,
H.An,
L.Govindasamy,
D.N.Silverman,
R.Mckenna,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 20.00 / 2.00 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 42.697, 41.620, 72.831, 90.00, 104.49, 90.00 |
| R / Rfree (%) | 13.4 / 17.5 |
Zinc Binding Sites:
The binding sites of Zinc atom in the Effect of Shuttle Location and pH Environment on H+ Transfer in Human Carbonic Anhydrase II
(pdb code 1teu). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total only one binding site of Zinc was determined in the Effect of Shuttle Location and pH Environment on H+ Transfer in Human Carbonic Anhydrase II, PDB code: 1teu:
In total only one binding site of Zinc was determined in the Effect of Shuttle Location and pH Environment on H+ Transfer in Human Carbonic Anhydrase II, PDB code: 1teu:
Zinc binding site 1 out of 1 in 1teu
Go back to
Zinc binding site 1 out
of 1 in the Effect of Shuttle Location and pH Environment on H+ Transfer in Human Carbonic Anhydrase II
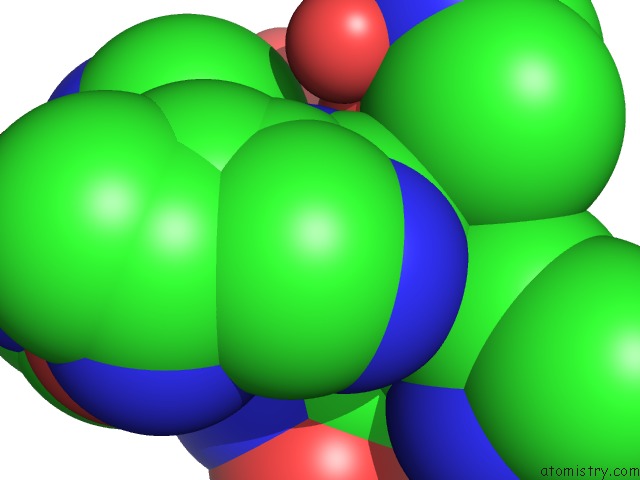
Mono view
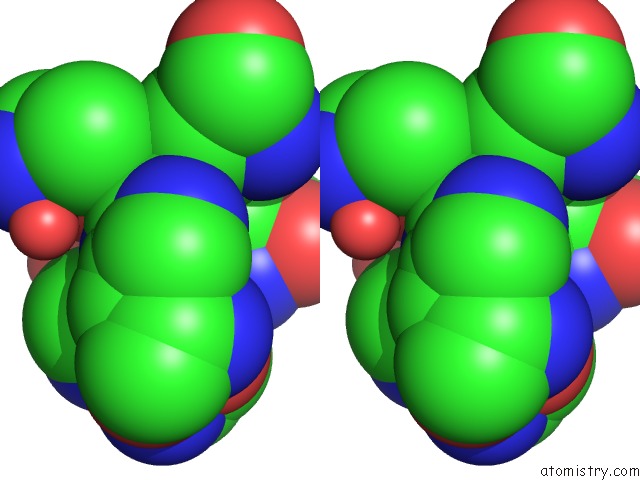
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Effect of Shuttle Location and pH Environment on H+ Transfer in Human Carbonic Anhydrase II within 5.0Å range:
|
Reference:
Z.Fisher,
J.A.Hernandez Prada,
C.K.Tu,
D.Duda,
C.Yoshioka,
H.An,
L.Govindasamy,
D.N.Silverman,
R.Mckenna.
Structural and Kinetic Characterization of Active-Site Histidine As A Proton Shuttle in Catalysis By Human Carbonic Anhydrase II Biochemistry V. 44 1097 2005.
ISSN: ISSN 0006-2960
PubMed: 15667203
DOI: 10.1021/BI0480279
Page generated: Tue Aug 19 23:19:22 2025
ISSN: ISSN 0006-2960
PubMed: 15667203
DOI: 10.1021/BI0480279
Last articles
Zn in 3DGDZn in 3DGN
Zn in 3DDT
Zn in 3DGM
Zn in 3DFM
Zn in 3DGL
Zn in 3DFX
Zn in 3DFV
Zn in 3DFK
Zn in 3DFF