Zinc »
PDB 1rag-1rqg »
1rk5 »
Zinc in PDB 1rk5: The D-Aminoacylase Mutant D366A in Complex with 100MM CUCL2
Enzymatic activity of The D-Aminoacylase Mutant D366A in Complex with 100MM CUCL2
All present enzymatic activity of The D-Aminoacylase Mutant D366A in Complex with 100MM CUCL2:
3.5.1.81;
3.5.1.81;
Protein crystallography data
The structure of The D-Aminoacylase Mutant D366A in Complex with 100MM CUCL2, PDB code: 1rk5
was solved by
W.L.Lai,
L.Y.Chou,
C.Y.Ting,
Y.C.Tsai,
S.H.Liaw,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 29.40 / 1.80 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 60.016, 77.266, 135.982, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 18.3 / 20.4 |
Other elements in 1rk5:
The structure of The D-Aminoacylase Mutant D366A in Complex with 100MM CUCL2 also contains other interesting chemical elements:
| Copper | (Cu) | 1 atom |
Zinc Binding Sites:
The binding sites of Zinc atom in the The D-Aminoacylase Mutant D366A in Complex with 100MM CUCL2
(pdb code 1rk5). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total only one binding site of Zinc was determined in the The D-Aminoacylase Mutant D366A in Complex with 100MM CUCL2, PDB code: 1rk5:
In total only one binding site of Zinc was determined in the The D-Aminoacylase Mutant D366A in Complex with 100MM CUCL2, PDB code: 1rk5:
Zinc binding site 1 out of 1 in 1rk5
Go back to
Zinc binding site 1 out
of 1 in the The D-Aminoacylase Mutant D366A in Complex with 100MM CUCL2
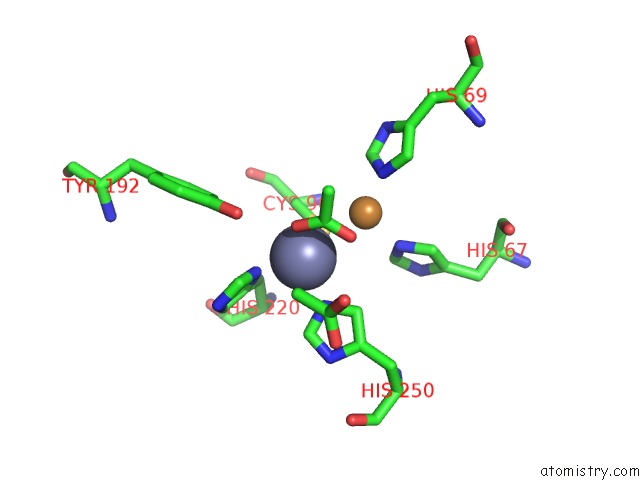
Mono view
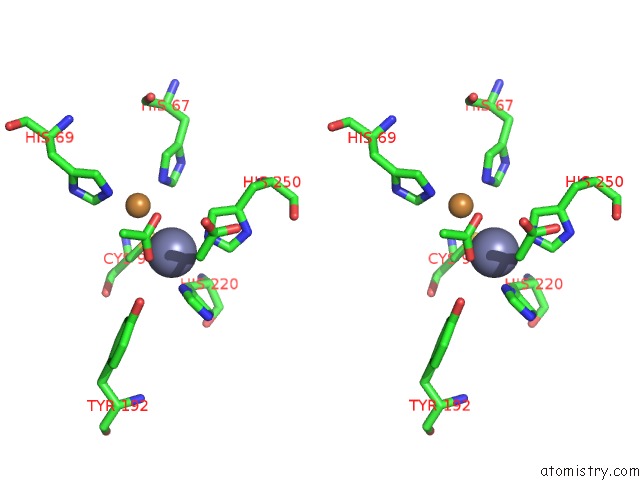
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of The D-Aminoacylase Mutant D366A in Complex with 100MM CUCL2 within 5.0Å range:
|
Reference:
W.L.Lai,
L.Y.Chou,
C.Y.Ting,
R.Kirby,
Y.C.Tsai,
A.H.Wang,
S.H.Liaw.
The Functional Role of the Binuclear Metal Center in D-Aminoacylase: One-Metal Activation and Second-Metal Attenuation. J.Biol.Chem. V. 279 13962 2004.
ISSN: ISSN 0021-9258
PubMed: 14736882
DOI: 10.1074/JBC.M308849200
Page generated: Tue Aug 19 22:57:00 2025
ISSN: ISSN 0021-9258
PubMed: 14736882
DOI: 10.1074/JBC.M308849200
Last articles
Zn in 2B0PZn in 2AZ4
Zn in 2AZH
Zn in 2AYS
Zn in 2AYK
Zn in 2AXR
Zn in 2AYJ
Zn in 2AYD
Zn in 2AVU
Zn in 2AX2