Zinc »
PDB 1fpp-1g43 »
1fql »
Zinc in PDB 1fql: X-Ray Crystal Structure of Zinc-Bound F95M/W97V Carbonic Anhydrase (Caii) Variant
Enzymatic activity of X-Ray Crystal Structure of Zinc-Bound F95M/W97V Carbonic Anhydrase (Caii) Variant
All present enzymatic activity of X-Ray Crystal Structure of Zinc-Bound F95M/W97V Carbonic Anhydrase (Caii) Variant:
4.2.1.1;
4.2.1.1;
Protein crystallography data
The structure of X-Ray Crystal Structure of Zinc-Bound F95M/W97V Carbonic Anhydrase (Caii) Variant, PDB code: 1fql
was solved by
J.D.Cox,
J.A.Hunt,
K.M.Compher,
C.A.Fierke,
D.W.Christianson,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 20.00 / 2.00 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 42.600, 41.900, 72.700, 90.00, 104.00, 90.00 |
| R / Rfree (%) | 17.9 / 25.5 |
Other elements in 1fql:
The structure of X-Ray Crystal Structure of Zinc-Bound F95M/W97V Carbonic Anhydrase (Caii) Variant also contains other interesting chemical elements:
| Mercury | (Hg) | 1 atom |
Zinc Binding Sites:
The binding sites of Zinc atom in the X-Ray Crystal Structure of Zinc-Bound F95M/W97V Carbonic Anhydrase (Caii) Variant
(pdb code 1fql). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total only one binding site of Zinc was determined in the X-Ray Crystal Structure of Zinc-Bound F95M/W97V Carbonic Anhydrase (Caii) Variant, PDB code: 1fql:
In total only one binding site of Zinc was determined in the X-Ray Crystal Structure of Zinc-Bound F95M/W97V Carbonic Anhydrase (Caii) Variant, PDB code: 1fql:
Zinc binding site 1 out of 1 in 1fql
Go back to
Zinc binding site 1 out
of 1 in the X-Ray Crystal Structure of Zinc-Bound F95M/W97V Carbonic Anhydrase (Caii) Variant

Mono view
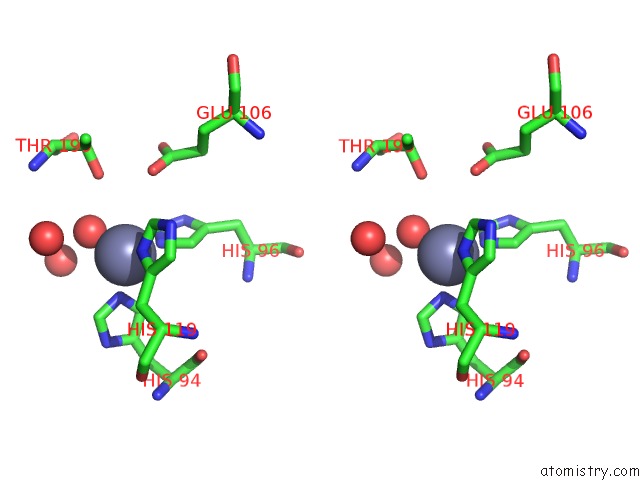
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of X-Ray Crystal Structure of Zinc-Bound F95M/W97V Carbonic Anhydrase (Caii) Variant within 5.0Å range:
|
Reference:
J.D.Cox,
J.A.Hunt,
K.M.Compher,
C.A.Fierke,
D.W.Christianson.
Structural Influence of Hydrophobic Core Residues on Metal Binding and Specificity in Carbonic Anhydrase II. Biochemistry V. 39 13687 2000.
ISSN: ISSN 0006-2960
PubMed: 11076507
DOI: 10.1021/BI001649J
Page generated: Sun Oct 13 01:05:32 2024
ISSN: ISSN 0006-2960
PubMed: 11076507
DOI: 10.1021/BI001649J
Last articles
Ag in 7SDJAg in 7SDH
Ag in 7ECK
Ag in 7SDF
Ag in 7EDN
Ag in 7ECP
Ag in 7CGD
Ag in 7ECO
Ag in 7ECN
Ag in 7CGC