Zinc »
PDB 9ntg-9ueq »
9q88 »
Zinc in PDB 9q88: High-Resolution Structure of RNF38 Ring Domain
Enzymatic activity of High-Resolution Structure of RNF38 Ring Domain
All present enzymatic activity of High-Resolution Structure of RNF38 Ring Domain:
2.3.2.27;
2.3.2.27;
Protein crystallography data
The structure of High-Resolution Structure of RNF38 Ring Domain, PDB code: 9q88
was solved by
M.Gabrielsen,
L.Buetow,
D.T.Huang,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 31.62 / 1.20 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 31.82, 39.309, 53.208, 90, 90, 90 |
| R / Rfree (%) | 15.1 / 16.8 |
Zinc Binding Sites:
The binding sites of Zinc atom in the High-Resolution Structure of RNF38 Ring Domain
(pdb code 9q88). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 2 binding sites of Zinc where determined in the High-Resolution Structure of RNF38 Ring Domain, PDB code: 9q88:
Jump to Zinc binding site number: 1; 2;
In total 2 binding sites of Zinc where determined in the High-Resolution Structure of RNF38 Ring Domain, PDB code: 9q88:
Jump to Zinc binding site number: 1; 2;
Zinc binding site 1 out of 2 in 9q88
Go back to
Zinc binding site 1 out
of 2 in the High-Resolution Structure of RNF38 Ring Domain
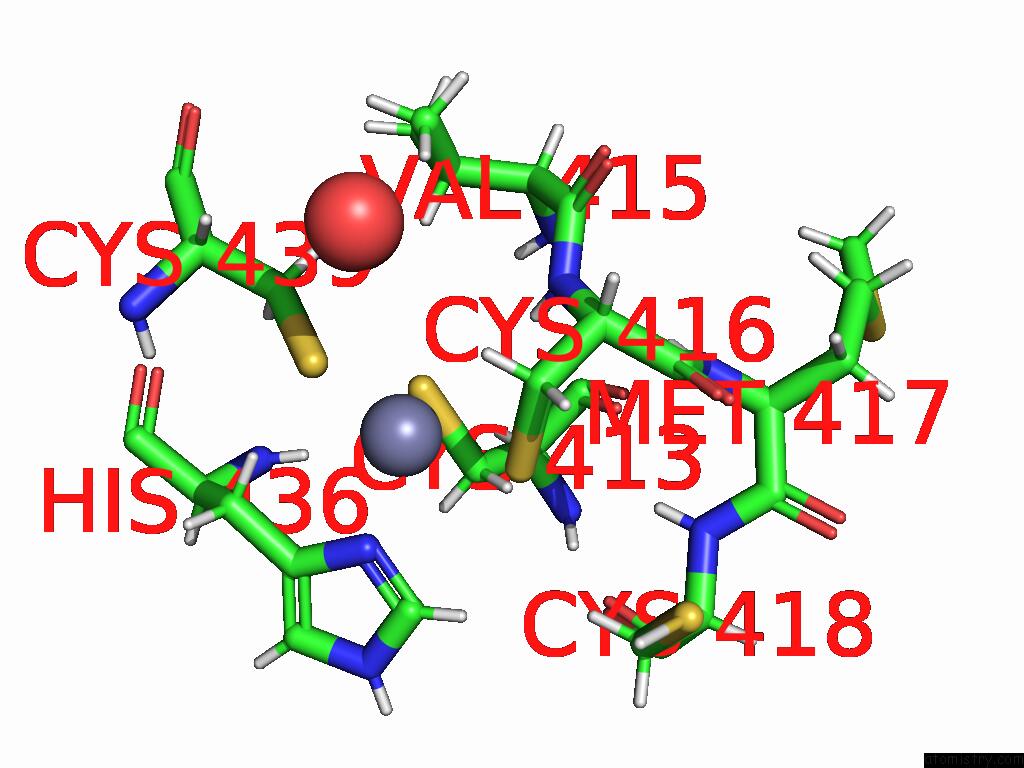
Mono view
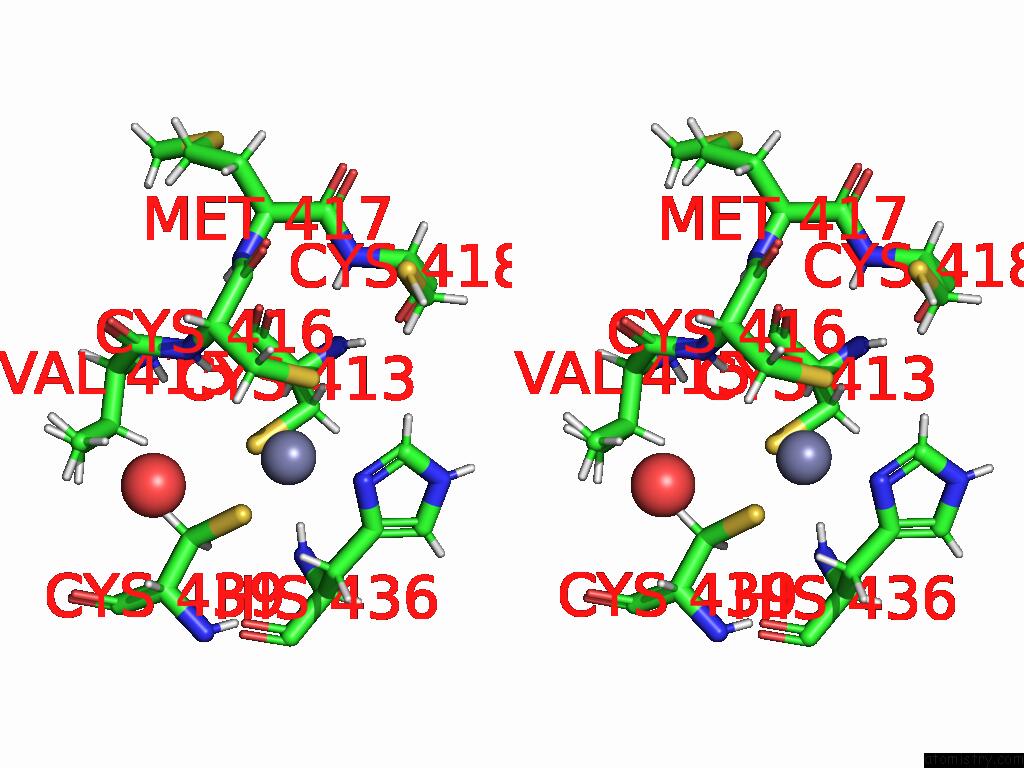
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of High-Resolution Structure of RNF38 Ring Domain within 5.0Å range:
|
Zinc binding site 2 out of 2 in 9q88
Go back to
Zinc binding site 2 out
of 2 in the High-Resolution Structure of RNF38 Ring Domain
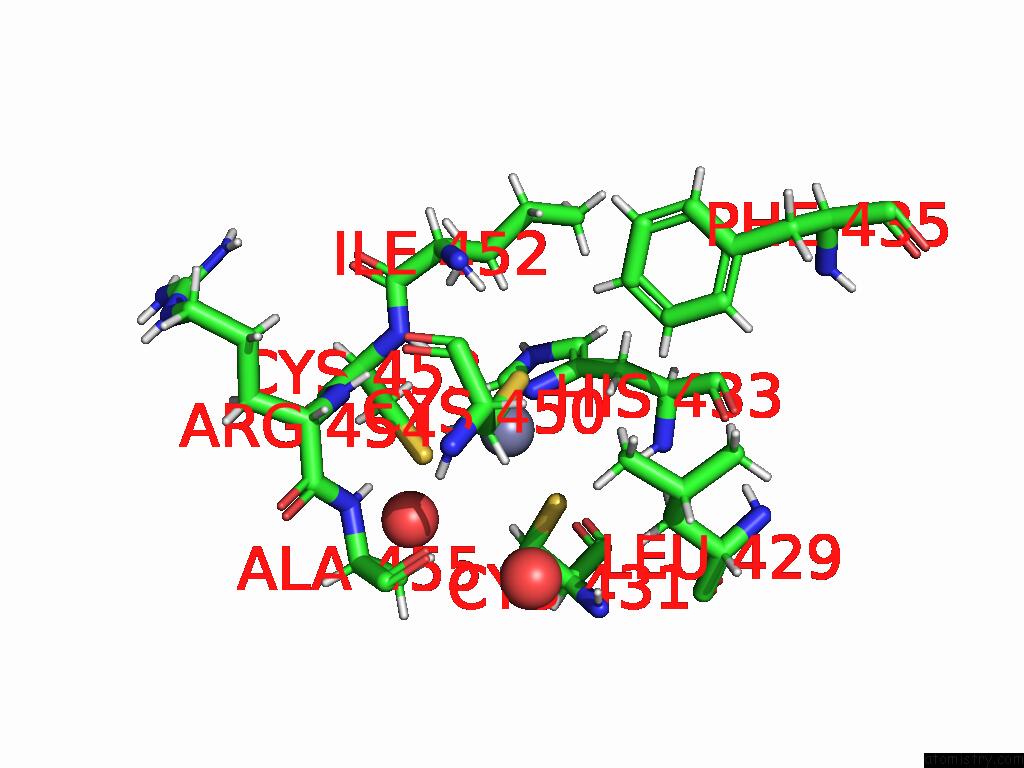
Mono view
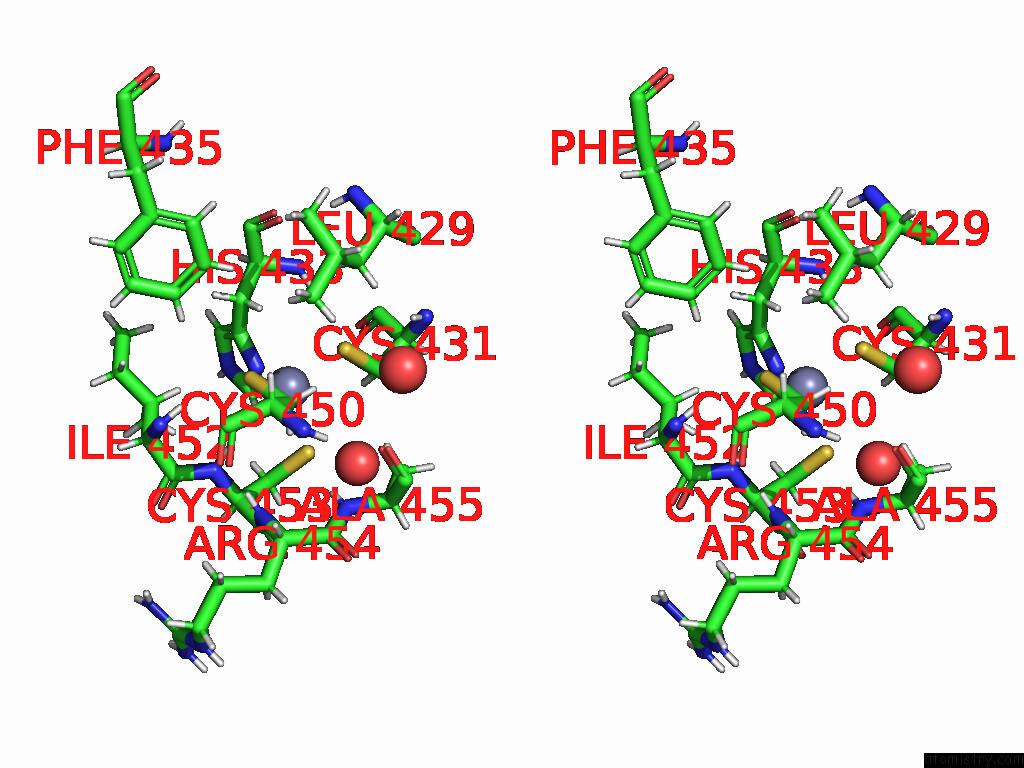
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of High-Resolution Structure of RNF38 Ring Domain within 5.0Å range:
|
Reference:
M.A.Nakasone,
L.Buetow,
M.Gabrielsen,
S.F.Ahmed,
K.Majorek,
G.J.Sibbet,
B.O.Smith,
D.T.Huang.
Tuning Ubiquitin Transfer By Ring E3 Ubiquitin Ligases Through the Linchpin Residue To Be Published.
Page generated: Fri Aug 22 18:52:58 2025
Last articles
Cd in 9J00Cd in 9J01
Ca in 9VAK
Ca in 9O9V
Ca in 9U8G
Ca in 9UD8
Ca in 9R0Q
Ca in 9QDT
Ca in 9O4Q
Ca in 9O4O