Zinc »
PDB 7c3s-7ci4 »
7cf6 »
Zinc in PDB 7cf6: Crystal Structure of Beta-Aspartyl Dipeptidase From Thermophilic Keratin Degrading Fervidobacterium Islandicum Aw-1 in Complex with Beta-Asp-Leu Dipeptide
Protein crystallography data
The structure of Crystal Structure of Beta-Aspartyl Dipeptidase From Thermophilic Keratin Degrading Fervidobacterium Islandicum Aw-1 in Complex with Beta-Asp-Leu Dipeptide, PDB code: 7cf6
was solved by
I.Dhanasingh,
J.W.La,
D.W.Lee,
S.H.Lee,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 47.66 / 2.75 |
| Space group | P 2 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 78.609, 150.425, 151.996, 90, 90, 90 |
| R / Rfree (%) | 19.6 / 27.8 |
Zinc Binding Sites:
The binding sites of Zinc atom in the Crystal Structure of Beta-Aspartyl Dipeptidase From Thermophilic Keratin Degrading Fervidobacterium Islandicum Aw-1 in Complex with Beta-Asp-Leu Dipeptide
(pdb code 7cf6). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 8 binding sites of Zinc where determined in the Crystal Structure of Beta-Aspartyl Dipeptidase From Thermophilic Keratin Degrading Fervidobacterium Islandicum Aw-1 in Complex with Beta-Asp-Leu Dipeptide, PDB code: 7cf6:
Jump to Zinc binding site number: 1; 2; 3; 4; 5; 6; 7; 8;
In total 8 binding sites of Zinc where determined in the Crystal Structure of Beta-Aspartyl Dipeptidase From Thermophilic Keratin Degrading Fervidobacterium Islandicum Aw-1 in Complex with Beta-Asp-Leu Dipeptide, PDB code: 7cf6:
Jump to Zinc binding site number: 1; 2; 3; 4; 5; 6; 7; 8;
Zinc binding site 1 out of 8 in 7cf6
Go back to
Zinc binding site 1 out
of 8 in the Crystal Structure of Beta-Aspartyl Dipeptidase From Thermophilic Keratin Degrading Fervidobacterium Islandicum Aw-1 in Complex with Beta-Asp-Leu Dipeptide
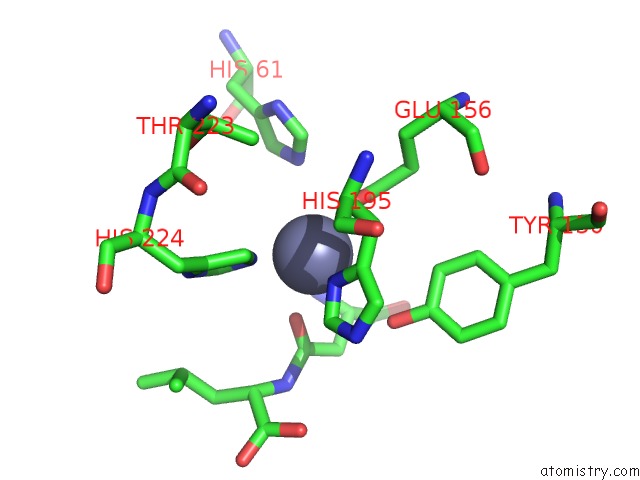
Mono view
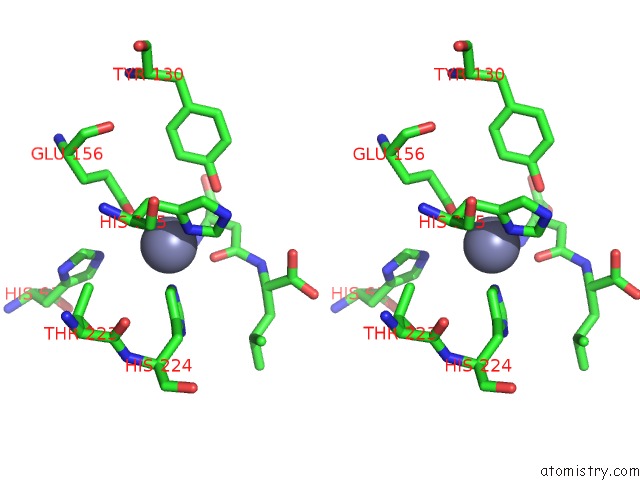
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Crystal Structure of Beta-Aspartyl Dipeptidase From Thermophilic Keratin Degrading Fervidobacterium Islandicum Aw-1 in Complex with Beta-Asp-Leu Dipeptide within 5.0Å range:
|
Zinc binding site 2 out of 8 in 7cf6
Go back to
Zinc binding site 2 out
of 8 in the Crystal Structure of Beta-Aspartyl Dipeptidase From Thermophilic Keratin Degrading Fervidobacterium Islandicum Aw-1 in Complex with Beta-Asp-Leu Dipeptide
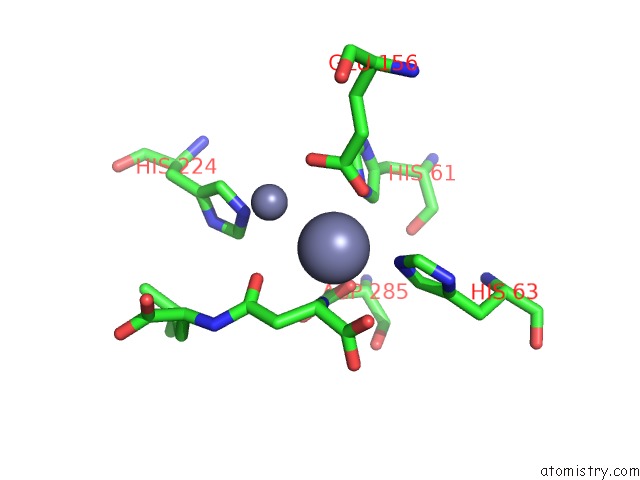
Mono view
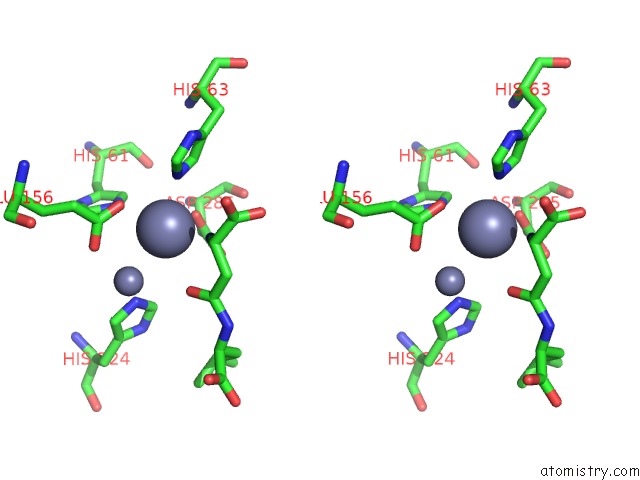
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of Crystal Structure of Beta-Aspartyl Dipeptidase From Thermophilic Keratin Degrading Fervidobacterium Islandicum Aw-1 in Complex with Beta-Asp-Leu Dipeptide within 5.0Å range:
|
Zinc binding site 3 out of 8 in 7cf6
Go back to
Zinc binding site 3 out
of 8 in the Crystal Structure of Beta-Aspartyl Dipeptidase From Thermophilic Keratin Degrading Fervidobacterium Islandicum Aw-1 in Complex with Beta-Asp-Leu Dipeptide
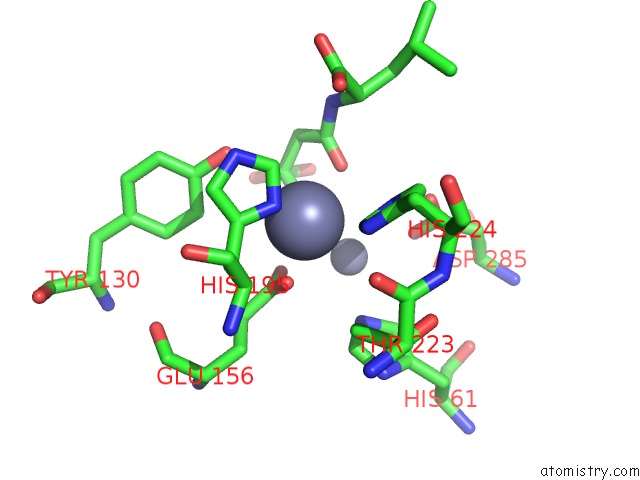
Mono view
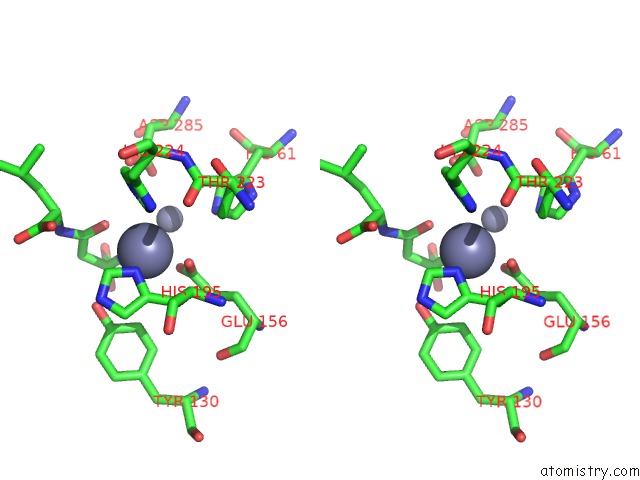
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 3 of Crystal Structure of Beta-Aspartyl Dipeptidase From Thermophilic Keratin Degrading Fervidobacterium Islandicum Aw-1 in Complex with Beta-Asp-Leu Dipeptide within 5.0Å range:
|
Zinc binding site 4 out of 8 in 7cf6
Go back to
Zinc binding site 4 out
of 8 in the Crystal Structure of Beta-Aspartyl Dipeptidase From Thermophilic Keratin Degrading Fervidobacterium Islandicum Aw-1 in Complex with Beta-Asp-Leu Dipeptide
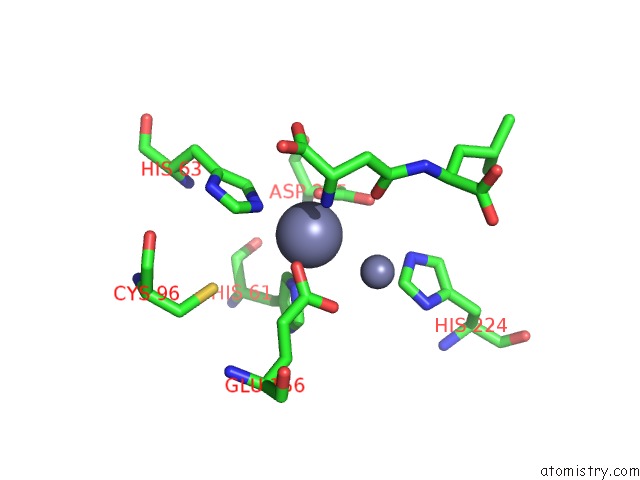
Mono view
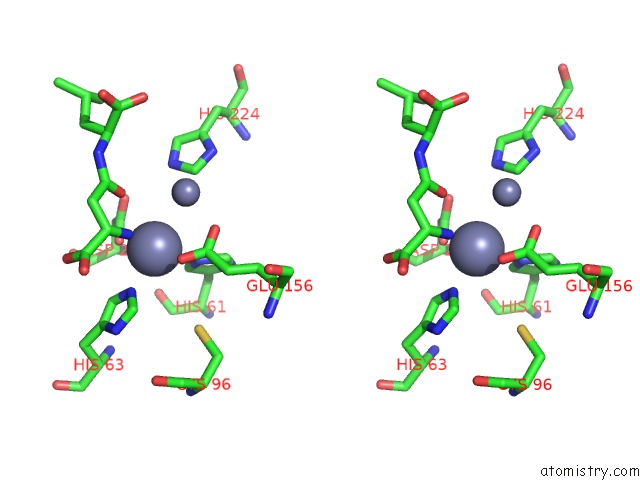
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 4 of Crystal Structure of Beta-Aspartyl Dipeptidase From Thermophilic Keratin Degrading Fervidobacterium Islandicum Aw-1 in Complex with Beta-Asp-Leu Dipeptide within 5.0Å range:
|
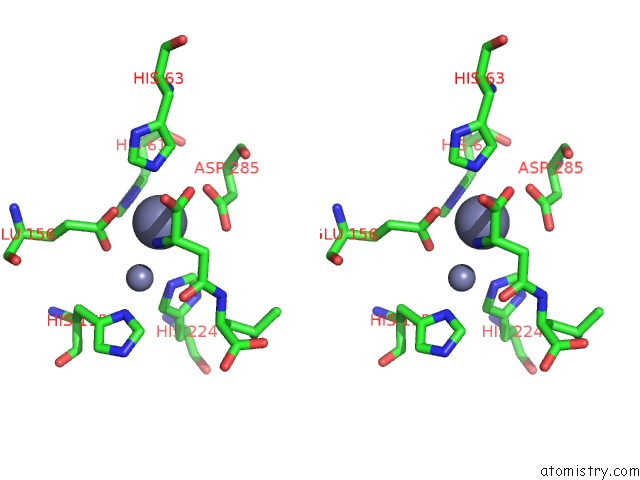
Zinc binding site 5 out of 8 in 7cf6
Go back to
Zinc binding site 5 out
of 8 in the Crystal Structure of Beta-Aspartyl Dipeptidase From Thermophilic Keratin Degrading Fervidobacterium Islandicum Aw-1 in Complex with Beta-Asp-Leu Dipeptide
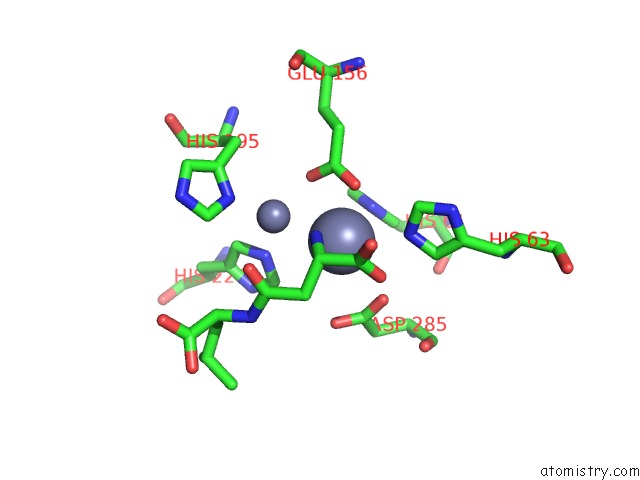
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 5 of Crystal Structure of Beta-Aspartyl Dipeptidase From Thermophilic Keratin Degrading Fervidobacterium Islandicum Aw-1 in Complex with Beta-Asp-Leu Dipeptide within 5.0Å range:
|
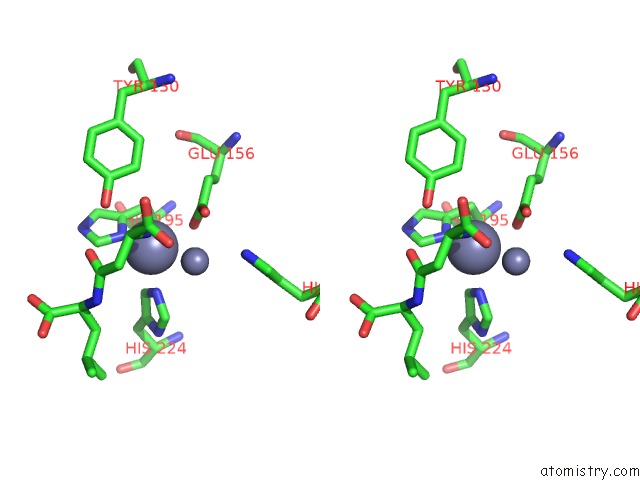
Zinc binding site 6 out of 8 in 7cf6
Go back to
Zinc binding site 6 out
of 8 in the Crystal Structure of Beta-Aspartyl Dipeptidase From Thermophilic Keratin Degrading Fervidobacterium Islandicum Aw-1 in Complex with Beta-Asp-Leu Dipeptide
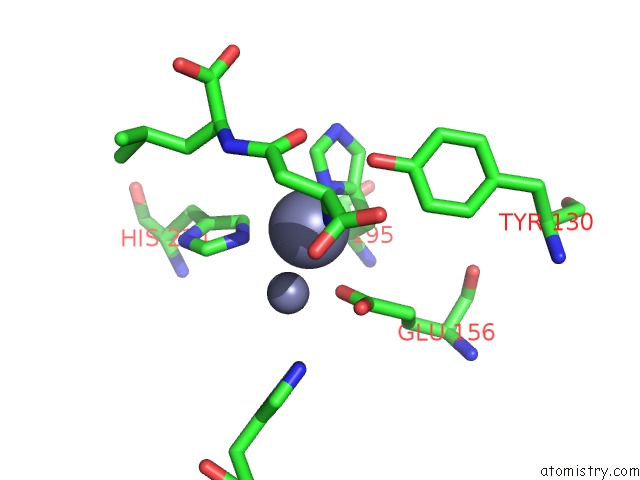
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 6 of Crystal Structure of Beta-Aspartyl Dipeptidase From Thermophilic Keratin Degrading Fervidobacterium Islandicum Aw-1 in Complex with Beta-Asp-Leu Dipeptide within 5.0Å range:
|
Zinc binding site 7 out of 8 in 7cf6
Go back to
Zinc binding site 7 out
of 8 in the Crystal Structure of Beta-Aspartyl Dipeptidase From Thermophilic Keratin Degrading Fervidobacterium Islandicum Aw-1 in Complex with Beta-Asp-Leu Dipeptide
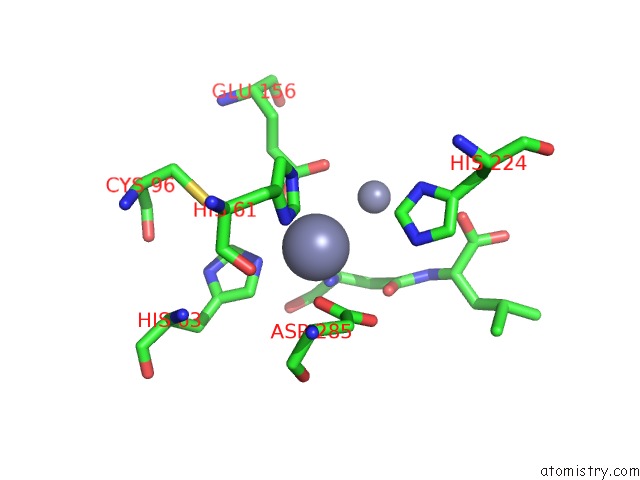
Mono view
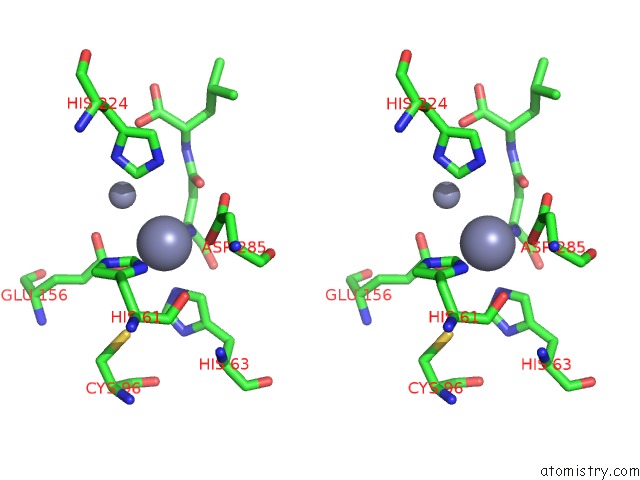
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 7 of Crystal Structure of Beta-Aspartyl Dipeptidase From Thermophilic Keratin Degrading Fervidobacterium Islandicum Aw-1 in Complex with Beta-Asp-Leu Dipeptide within 5.0Å range:
|
Zinc binding site 8 out of 8 in 7cf6
Go back to
Zinc binding site 8 out
of 8 in the Crystal Structure of Beta-Aspartyl Dipeptidase From Thermophilic Keratin Degrading Fervidobacterium Islandicum Aw-1 in Complex with Beta-Asp-Leu Dipeptide
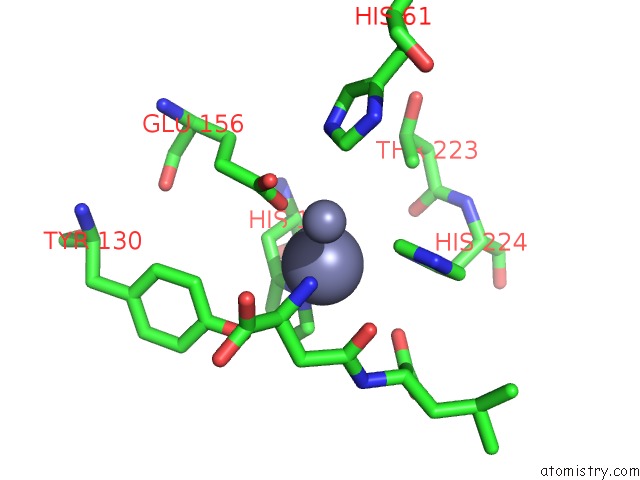
Mono view
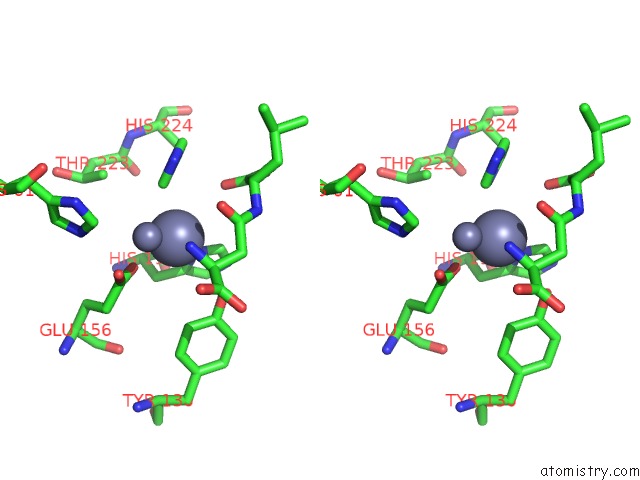
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 8 of Crystal Structure of Beta-Aspartyl Dipeptidase From Thermophilic Keratin Degrading Fervidobacterium Islandicum Aw-1 in Complex with Beta-Asp-Leu Dipeptide within 5.0Å range:
|
Reference:
J.W.La,
I.Dhanasingh,
H.Jang,
S.H.Lee,
D.W.Lee.
Functional Characterization of Primordial Protein Repair Enzyme M38 Metallo-Peptidase From Fervidobacterium Islandicum Aw-1. Front Mol Biosci V. 7 00634 2020.
ISSN: ESSN 2296-889X
PubMed: 33392259
DOI: 10.3389/FMOLB.2020.600634
Page generated: Tue Oct 29 18:11:41 2024
ISSN: ESSN 2296-889X
PubMed: 33392259
DOI: 10.3389/FMOLB.2020.600634
Last articles
K in 7EAYK in 7E20
K in 7DVQ
K in 7D5E
K in 7DMR
K in 7DIF
K in 7COW
K in 7D5D
K in 7D99
K in 7D52