Zinc »
PDB 6mby-6mn4 »
6mdw »
Zinc in PDB 6mdw: Mechanism of Protease Dependent Dpc Repair
Protein crystallography data
The structure of Mechanism of Protease Dependent Dpc Repair, PDB code: 6mdw
was solved by
F.Li,
J.Raczynska,
Z.Chen,
H.Yu,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 32.58 / 1.50 |
| Space group | P 21 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 128.422, 29.553, 50.242, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 16.3 / 19.3 |
Zinc Binding Sites:
The binding sites of Zinc atom in the Mechanism of Protease Dependent Dpc Repair
(pdb code 6mdw). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 3 binding sites of Zinc where determined in the Mechanism of Protease Dependent Dpc Repair, PDB code: 6mdw:
Jump to Zinc binding site number: 1; 2; 3;
In total 3 binding sites of Zinc where determined in the Mechanism of Protease Dependent Dpc Repair, PDB code: 6mdw:
Jump to Zinc binding site number: 1; 2; 3;
Zinc binding site 1 out of 3 in 6mdw
Go back to
Zinc binding site 1 out
of 3 in the Mechanism of Protease Dependent Dpc Repair
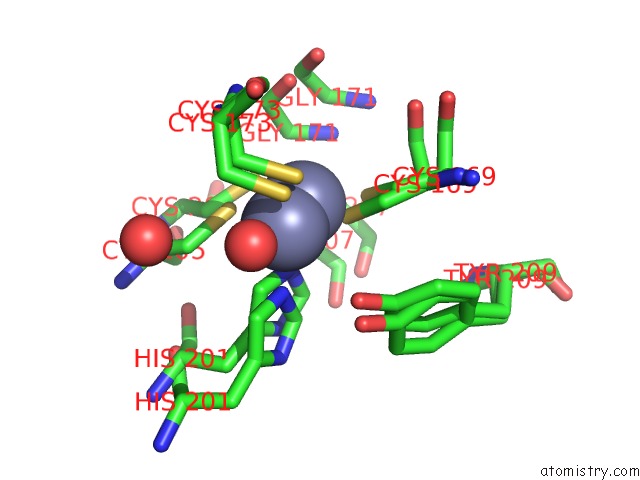
Mono view
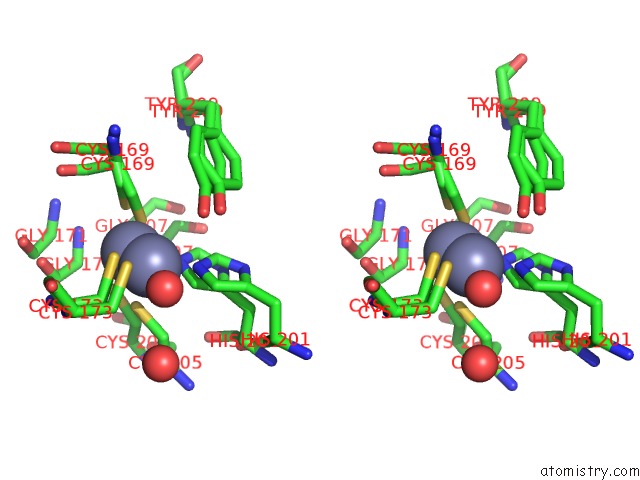
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Mechanism of Protease Dependent Dpc Repair within 5.0Å range:
|
Zinc binding site 2 out of 3 in 6mdw
Go back to
Zinc binding site 2 out
of 3 in the Mechanism of Protease Dependent Dpc Repair
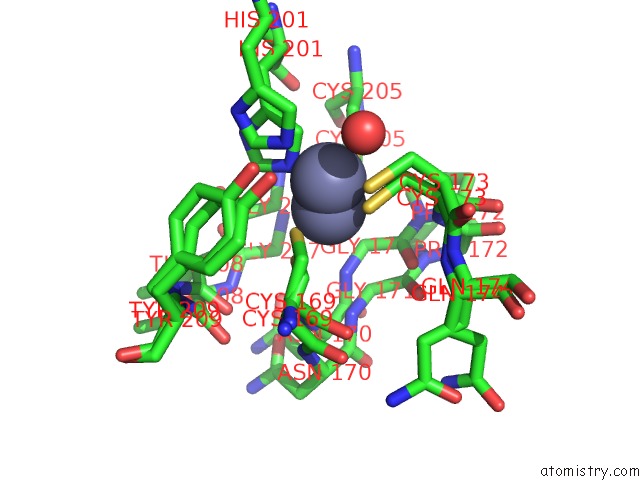
Mono view
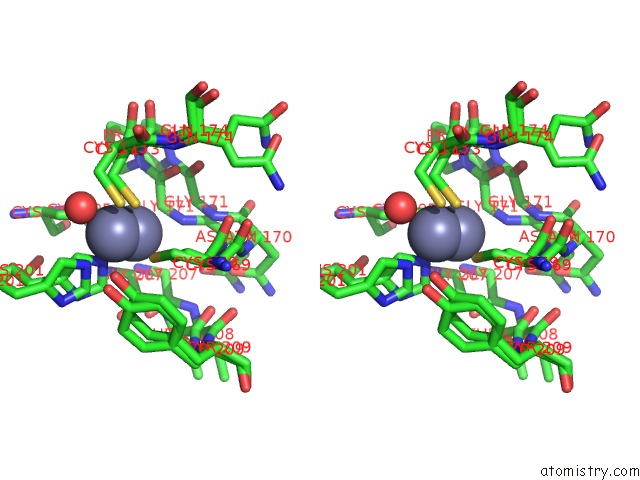
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of Mechanism of Protease Dependent Dpc Repair within 5.0Å range:
|
Zinc binding site 3 out of 3 in 6mdw
Go back to
Zinc binding site 3 out
of 3 in the Mechanism of Protease Dependent Dpc Repair
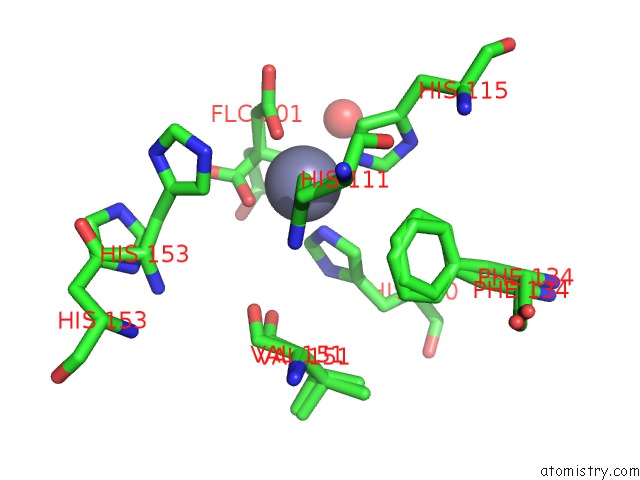
Mono view
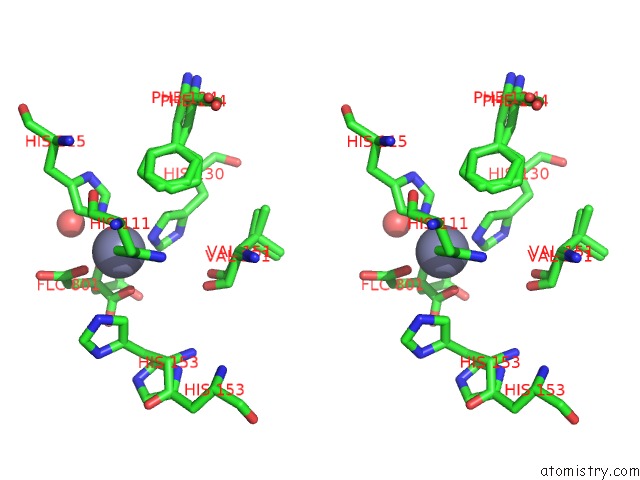
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 3 of Mechanism of Protease Dependent Dpc Repair within 5.0Å range:
|
Reference:
F.Li,
J.E.Raczynska,
Z.Chen,
H.Yu.
Structural Insight Into Dna-Dependent Activation of Human Metalloprotease Spartan. Cell Rep V. 26 3336 2019.
ISSN: ESSN 2211-1247
PubMed: 30893605
DOI: 10.1016/J.CELREP.2019.02.082
Page generated: Tue Oct 29 03:04:22 2024
ISSN: ESSN 2211-1247
PubMed: 30893605
DOI: 10.1016/J.CELREP.2019.02.082
Last articles
Zn in 5B15Zn in 5B0H
Zn in 5AWS
Zn in 5AYA
Zn in 5AYO
Zn in 5AXR
Zn in 5AXQ
Zn in 5AXP
Zn in 5AXO
Zn in 5AWT