Zinc »
PDB 6i0w-6iiw »
6i1d »
Zinc in PDB 6i1d: Structure of the YSH1-MPE1 Nuclease Complex From S.Cerevisiae
Protein crystallography data
The structure of Structure of the YSH1-MPE1 Nuclease Complex From S.Cerevisiae, PDB code: 6i1d
was solved by
C.H.Hill,
V.Boreikaite,
A.Kumar,
A.Casanal,
P.Kubik,
G.Degliesposti,
S.Maslen,
A.Mariani,
O.Von Loeffelholz,
M.Girbig,
M.Skehel,
L.A.Passmore,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 62.14 / 2.28 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 43.380, 124.270, 63.450, 90.00, 103.21, 90.00 |
| R / Rfree (%) | 17.3 / 22.2 |
Zinc Binding Sites:
The binding sites of Zinc atom in the Structure of the YSH1-MPE1 Nuclease Complex From S.Cerevisiae
(pdb code 6i1d). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 2 binding sites of Zinc where determined in the Structure of the YSH1-MPE1 Nuclease Complex From S.Cerevisiae, PDB code: 6i1d:
Jump to Zinc binding site number: 1; 2;
In total 2 binding sites of Zinc where determined in the Structure of the YSH1-MPE1 Nuclease Complex From S.Cerevisiae, PDB code: 6i1d:
Jump to Zinc binding site number: 1; 2;
Zinc binding site 1 out of 2 in 6i1d
Go back to
Zinc binding site 1 out
of 2 in the Structure of the YSH1-MPE1 Nuclease Complex From S.Cerevisiae
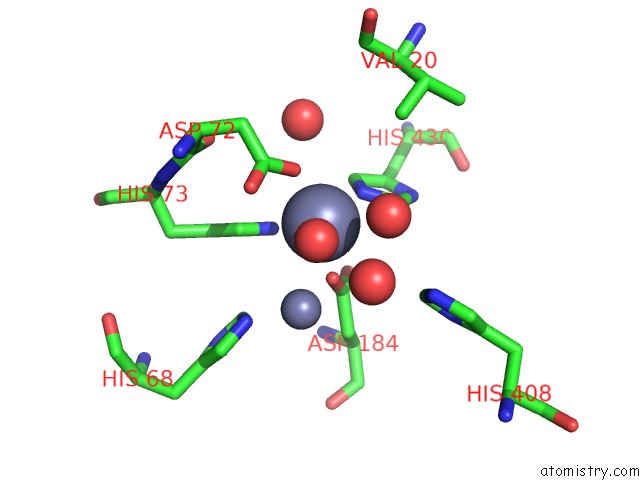
Mono view
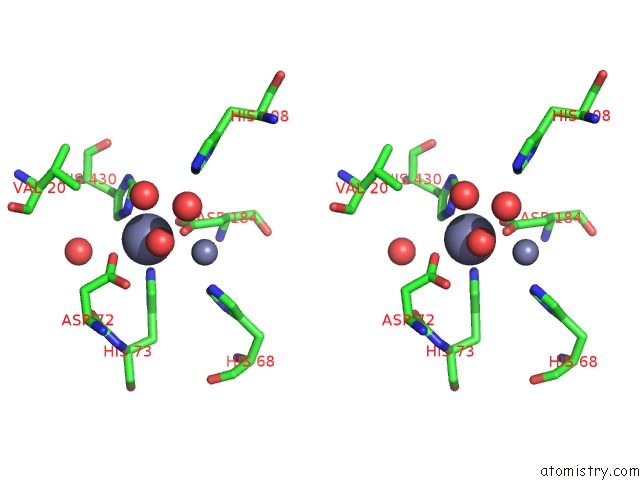
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Structure of the YSH1-MPE1 Nuclease Complex From S.Cerevisiae within 5.0Å range:
|
Zinc binding site 2 out of 2 in 6i1d
Go back to
Zinc binding site 2 out
of 2 in the Structure of the YSH1-MPE1 Nuclease Complex From S.Cerevisiae
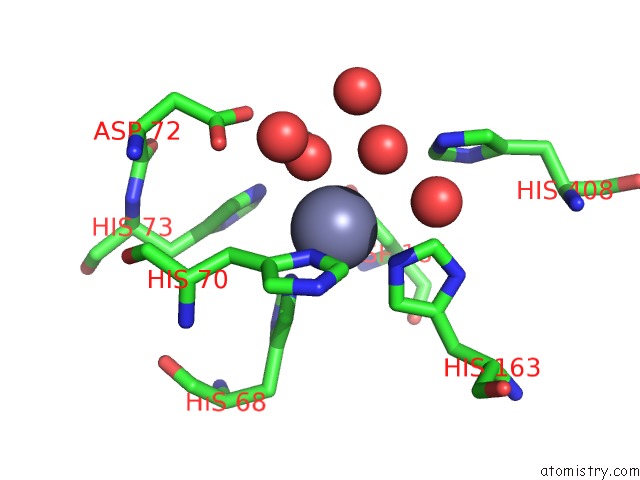
Mono view
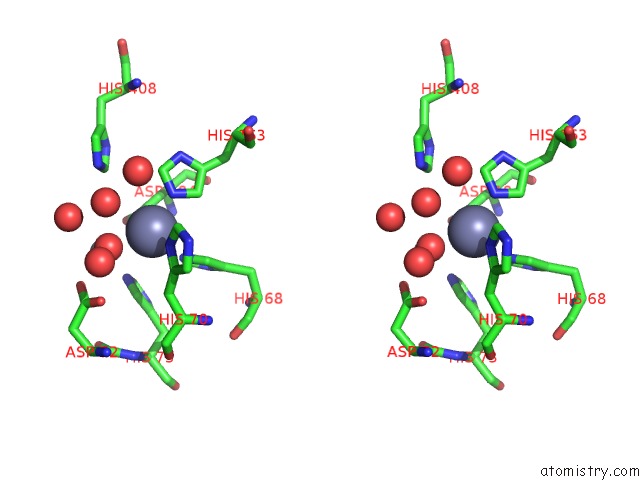
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of Structure of the YSH1-MPE1 Nuclease Complex From S.Cerevisiae within 5.0Å range:
|
Reference:
C.H.Hill,
V.Boreikaite,
A.Kumar,
A.Casanal,
P.Kubik,
G.Degliesposti,
S.Maslen,
A.Mariani,
O.Von Loeffelholz,
M.Girbig,
M.Skehel,
L.A.Passmore.
Activation of the Endonuclease That Defines Mrna 3' Ends Requires Incorporation Into An 8-Subunit Core Cleavage and Polyadenylation Factor Complex. Mol.Cell V. 73 1217 2019.
ISSN: ISSN 1097-2765
PubMed: 30737185
DOI: 10.1016/J.MOLCEL.2018.12.023
Page generated: Mon Oct 28 23:34:40 2024
ISSN: ISSN 1097-2765
PubMed: 30737185
DOI: 10.1016/J.MOLCEL.2018.12.023
Last articles
K in 5YFUK in 5YFT
K in 5XRZ
K in 5YFS
K in 5YEW
K in 5YFJ
K in 5YFN
K in 5Y7X
K in 5XUV
K in 5Y2P