Zinc »
PDB 5svc-5t72 »
5szb »
Zinc in PDB 5szb: Structure of Human DPF3 Double-Phd Domain Bound to Histone H3 Tail Peptide with Acetylated K14
Protein crystallography data
The structure of Structure of Human DPF3 Double-Phd Domain Bound to Histone H3 Tail Peptide with Acetylated K14, PDB code: 5szb
was solved by
N.Singh,
A.Local,
A.Shiau,
B.Ren,
K.D.Corbett,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 38.00 / 1.20 |
| Space group | P 43 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 39.321, 39.321, 147.571, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 13.8 / 15.5 |
Zinc Binding Sites:
The binding sites of Zinc atom in the Structure of Human DPF3 Double-Phd Domain Bound to Histone H3 Tail Peptide with Acetylated K14
(pdb code 5szb). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 4 binding sites of Zinc where determined in the Structure of Human DPF3 Double-Phd Domain Bound to Histone H3 Tail Peptide with Acetylated K14, PDB code: 5szb:
Jump to Zinc binding site number: 1; 2; 3; 4;
In total 4 binding sites of Zinc where determined in the Structure of Human DPF3 Double-Phd Domain Bound to Histone H3 Tail Peptide with Acetylated K14, PDB code: 5szb:
Jump to Zinc binding site number: 1; 2; 3; 4;
Zinc binding site 1 out of 4 in 5szb
Go back to
Zinc binding site 1 out
of 4 in the Structure of Human DPF3 Double-Phd Domain Bound to Histone H3 Tail Peptide with Acetylated K14
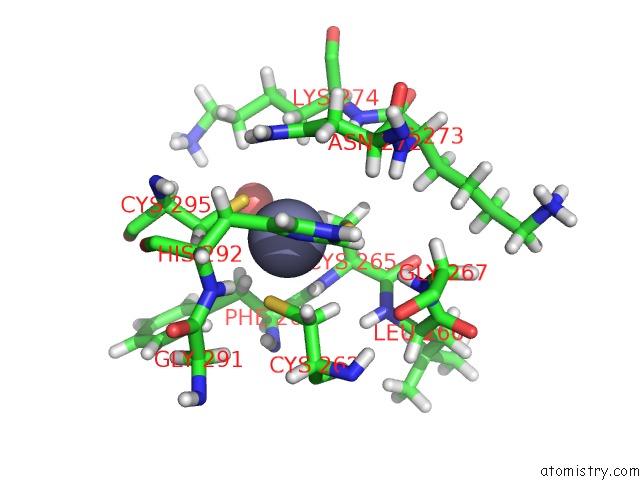
Mono view
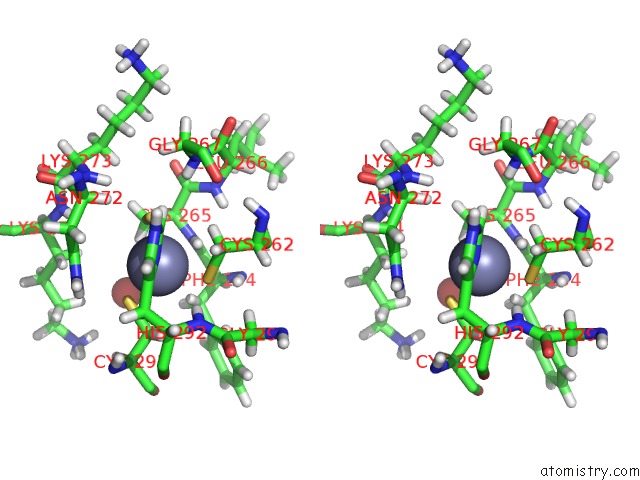
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Structure of Human DPF3 Double-Phd Domain Bound to Histone H3 Tail Peptide with Acetylated K14 within 5.0Å range:
|
Zinc binding site 2 out of 4 in 5szb
Go back to
Zinc binding site 2 out
of 4 in the Structure of Human DPF3 Double-Phd Domain Bound to Histone H3 Tail Peptide with Acetylated K14
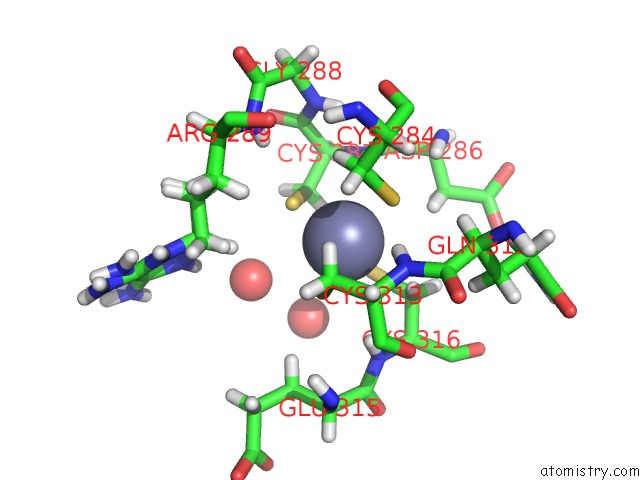
Mono view
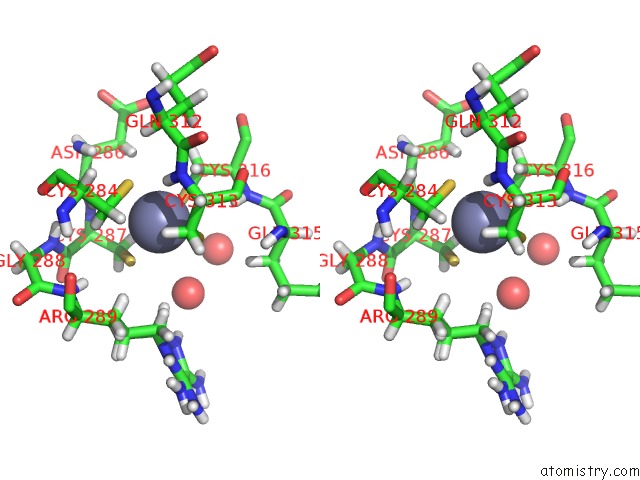
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of Structure of Human DPF3 Double-Phd Domain Bound to Histone H3 Tail Peptide with Acetylated K14 within 5.0Å range:
|
Zinc binding site 3 out of 4 in 5szb
Go back to
Zinc binding site 3 out
of 4 in the Structure of Human DPF3 Double-Phd Domain Bound to Histone H3 Tail Peptide with Acetylated K14
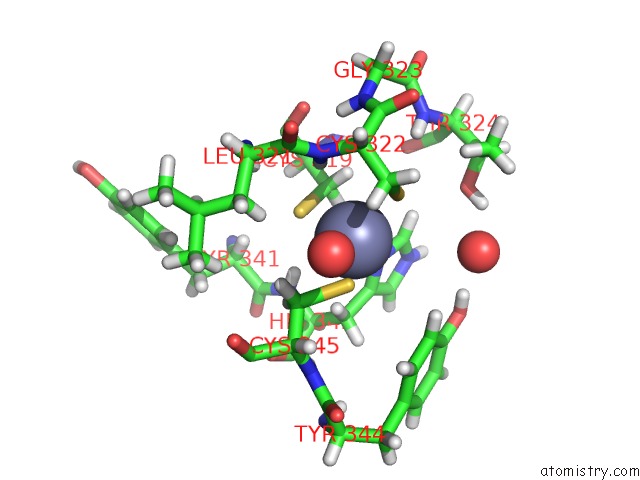
Mono view
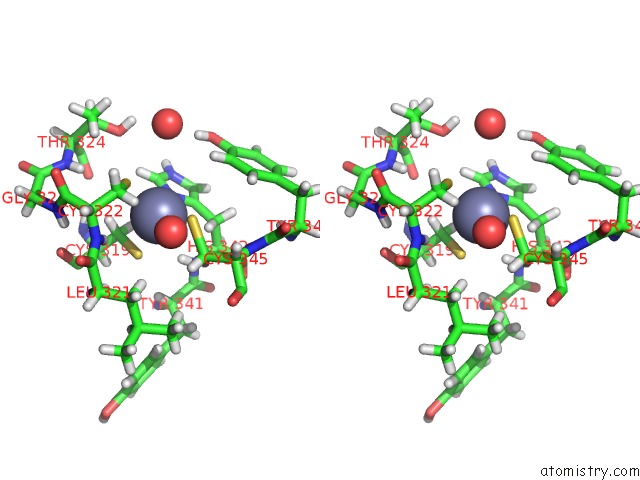
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 3 of Structure of Human DPF3 Double-Phd Domain Bound to Histone H3 Tail Peptide with Acetylated K14 within 5.0Å range:
|
Zinc binding site 4 out of 4 in 5szb
Go back to
Zinc binding site 4 out
of 4 in the Structure of Human DPF3 Double-Phd Domain Bound to Histone H3 Tail Peptide with Acetylated K14
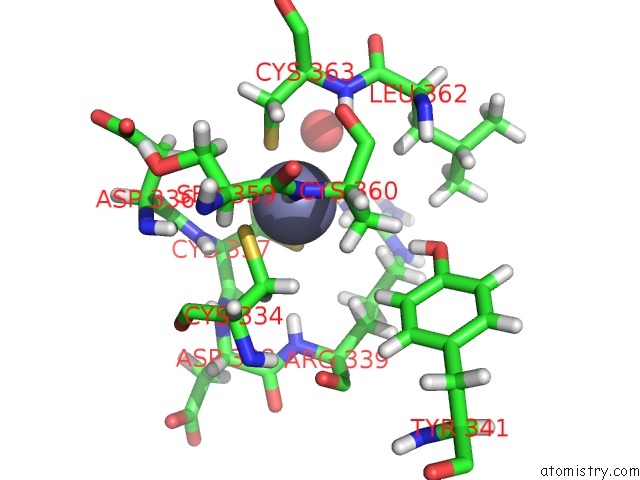
Mono view
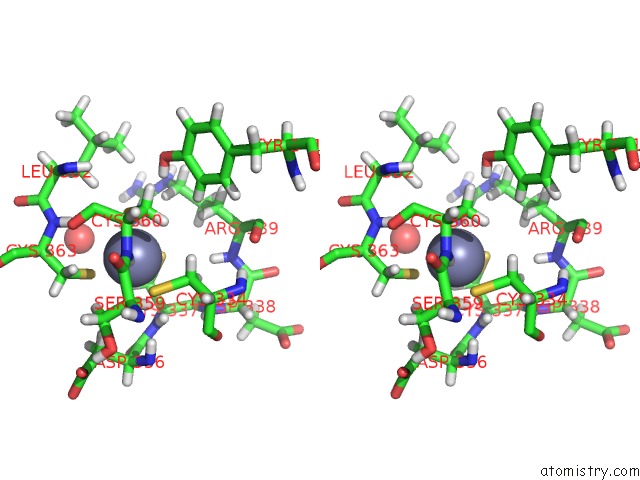
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 4 of Structure of Human DPF3 Double-Phd Domain Bound to Histone H3 Tail Peptide with Acetylated K14 within 5.0Å range:
|
Reference:
A.Local,
H.Huang,
C.P.Albuquerque,
N.Singh,
A.Y.Lee,
W.Wang,
C.Wang,
J.E.Hsia,
A.K.Shiau,
K.Ge,
K.D.Corbett,
D.Wang,
H.Zhou,
B.Ren.
Identification of H3K4ME1-Associated Proteins at Mammalian Enhancers. Nat. Genet. V. 50 73 2018.
ISSN: ISSN 1546-1718
PubMed: 29255264
DOI: 10.1038/S41588-017-0015-6
Page generated: Mon Oct 28 08:10:02 2024
ISSN: ISSN 1546-1718
PubMed: 29255264
DOI: 10.1038/S41588-017-0015-6
Last articles
Mg in 6DMDMg in 6DLS
Mg in 6DLT
Mg in 6DLU
Mg in 6DLR
Mg in 6DKO
Mg in 6DL9
Mg in 6DLJ
Mg in 6DJQ
Mg in 6DJO