Zinc »
PDB 5mt9-5n34 »
5n1p »
Zinc in PDB 5n1p: Crystal Structure of the Polysaccharide Deacetylase BC1974 From Bacillus Cereus in Complex with N-Hydroxynaphthalene-1-Carboxamide
Protein crystallography data
The structure of Crystal Structure of the Polysaccharide Deacetylase BC1974 From Bacillus Cereus in Complex with N-Hydroxynaphthalene-1-Carboxamide, PDB code: 5n1p
was solved by
P.Giastas,
A.Andreou,
E.E.Eliopoulos,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 48.45 / 1.45 |
| Space group | C 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 196.103, 44.347, 99.132, 90.00, 98.83, 90.00 |
| R / Rfree (%) | 16.1 / 18 |
Other elements in 5n1p:
The structure of Crystal Structure of the Polysaccharide Deacetylase BC1974 From Bacillus Cereus in Complex with N-Hydroxynaphthalene-1-Carboxamide also contains other interesting chemical elements:
| Sodium | (Na) | 2 atoms |
Zinc Binding Sites:
The binding sites of Zinc atom in the Crystal Structure of the Polysaccharide Deacetylase BC1974 From Bacillus Cereus in Complex with N-Hydroxynaphthalene-1-Carboxamide
(pdb code 5n1p). This binding sites where shown within
5.0 Angstroms radius around Zinc atom.
In total 4 binding sites of Zinc where determined in the Crystal Structure of the Polysaccharide Deacetylase BC1974 From Bacillus Cereus in Complex with N-Hydroxynaphthalene-1-Carboxamide, PDB code: 5n1p:
Jump to Zinc binding site number: 1; 2; 3; 4;
In total 4 binding sites of Zinc where determined in the Crystal Structure of the Polysaccharide Deacetylase BC1974 From Bacillus Cereus in Complex with N-Hydroxynaphthalene-1-Carboxamide, PDB code: 5n1p:
Jump to Zinc binding site number: 1; 2; 3; 4;
Zinc binding site 1 out of 4 in 5n1p
Go back to
Zinc binding site 1 out
of 4 in the Crystal Structure of the Polysaccharide Deacetylase BC1974 From Bacillus Cereus in Complex with N-Hydroxynaphthalene-1-Carboxamide
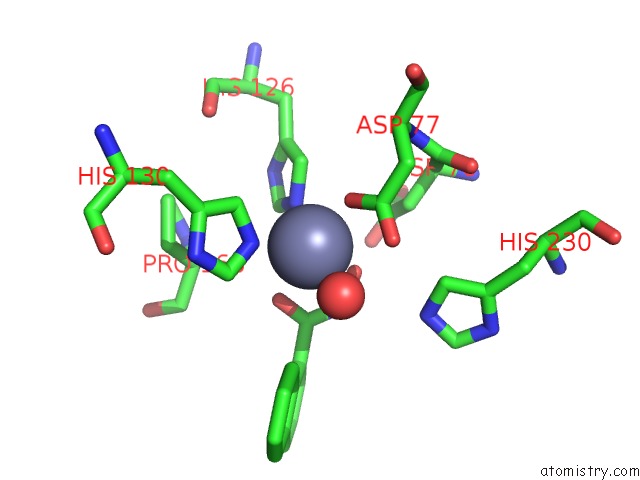
Mono view
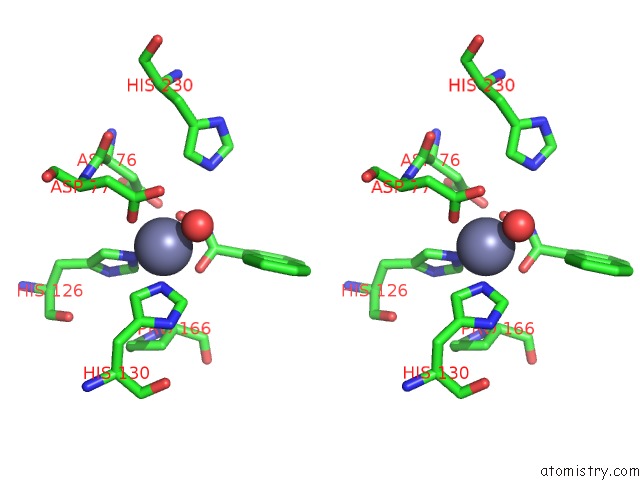
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 1 of Crystal Structure of the Polysaccharide Deacetylase BC1974 From Bacillus Cereus in Complex with N-Hydroxynaphthalene-1-Carboxamide within 5.0Å range:
|
Zinc binding site 2 out of 4 in 5n1p
Go back to
Zinc binding site 2 out
of 4 in the Crystal Structure of the Polysaccharide Deacetylase BC1974 From Bacillus Cereus in Complex with N-Hydroxynaphthalene-1-Carboxamide
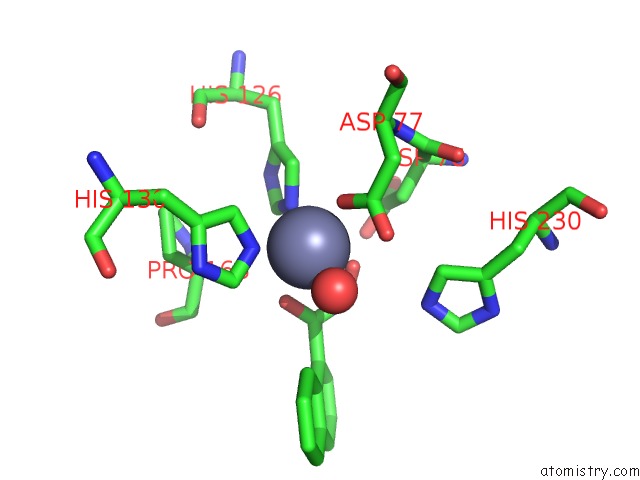
Mono view
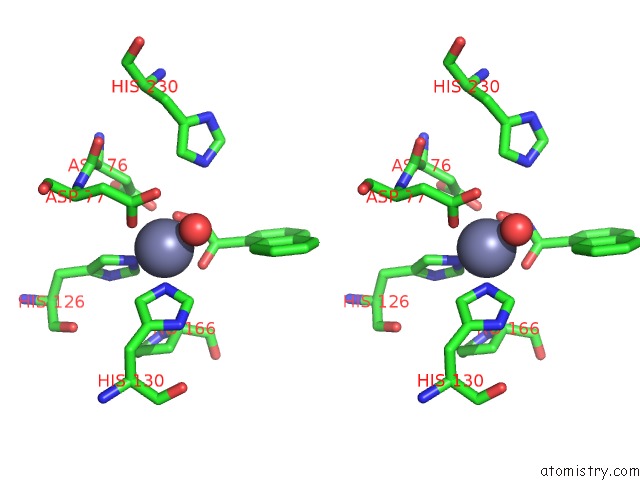
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 2 of Crystal Structure of the Polysaccharide Deacetylase BC1974 From Bacillus Cereus in Complex with N-Hydroxynaphthalene-1-Carboxamide within 5.0Å range:
|
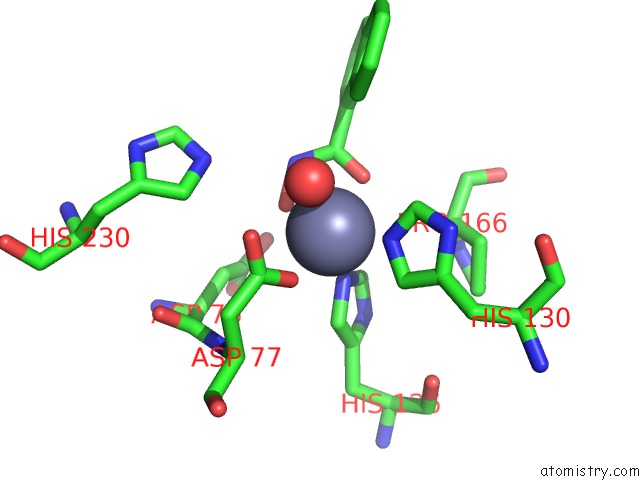
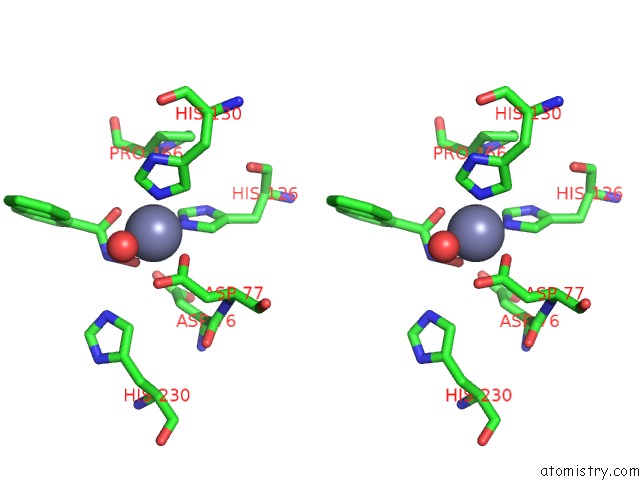
Zinc binding site 3 out of 4 in 5n1p
Go back to
Zinc binding site 3 out
of 4 in the Crystal Structure of the Polysaccharide Deacetylase BC1974 From Bacillus Cereus in Complex with N-Hydroxynaphthalene-1-Carboxamide
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 3 of Crystal Structure of the Polysaccharide Deacetylase BC1974 From Bacillus Cereus in Complex with N-Hydroxynaphthalene-1-Carboxamide within 5.0Å range:
|
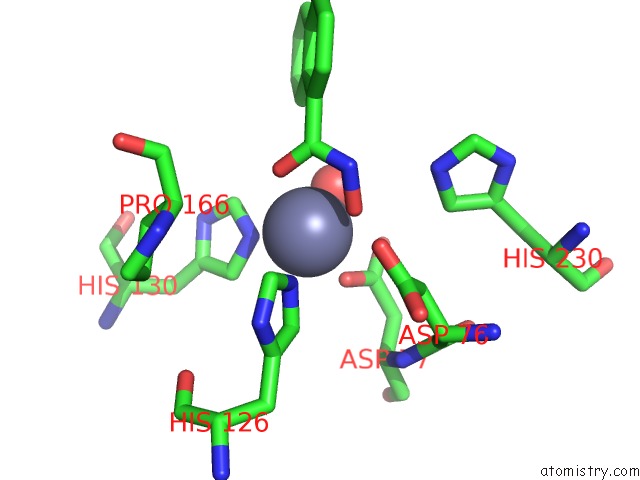
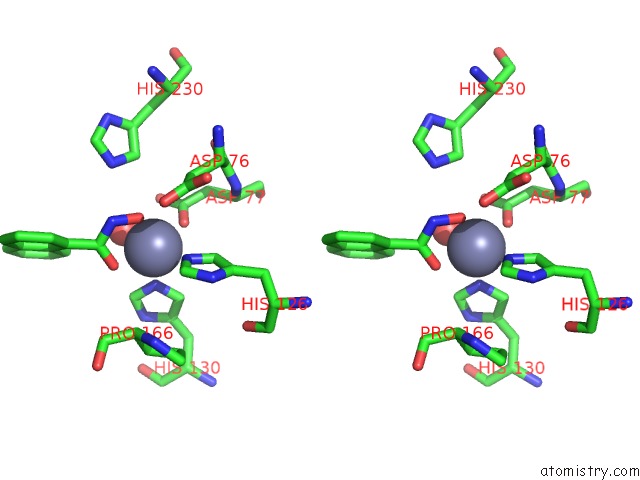
Zinc binding site 4 out of 4 in 5n1p
Go back to
Zinc binding site 4 out
of 4 in the Crystal Structure of the Polysaccharide Deacetylase BC1974 From Bacillus Cereus in Complex with N-Hydroxynaphthalene-1-Carboxamide
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Zinc with other atoms in the Zn binding
site number 4 of Crystal Structure of the Polysaccharide Deacetylase BC1974 From Bacillus Cereus in Complex with N-Hydroxynaphthalene-1-Carboxamide within 5.0Å range:
|
Reference:
P.Giastas,
A.Andreou,
A.Papakyriakou,
D.Koutsioulis,
S.Balomenou,
S.J.Tzartos,
V.Bouriotis,
E.E.Eliopoulos.
Structures of the Peptidoglycan N-Acetylglucosamine Deacetylase BC1974 and Its Complexes with Zinc Metalloenzyme Inhibitors. Biochemistry V. 57 753 2018.
ISSN: ISSN 1520-4995
PubMed: 29257674
DOI: 10.1021/ACS.BIOCHEM.7B00919
Page generated: Thu Aug 21 05:24:31 2025
ISSN: ISSN 1520-4995
PubMed: 29257674
DOI: 10.1021/ACS.BIOCHEM.7B00919
Last articles
Mn in 9LJUMn in 9LJW
Mn in 9LJS
Mn in 9LJR
Mn in 9LJT
Mn in 9LJV
Mg in 9UA2
Mg in 9R96
Mg in 9VM1
Mg in 9P01